Intrinsic Regulation of Hepatic Blood Flow
The control of hepatic blood flow involves both intrinsic and
extrinsic mechanisms. Intrinsic regulation, which works independently of neurohumoral
influences, includes pressure-flow autoregulation, metabolic control, and the hepatic
arterial buffer response.
Pressure-Flow Autoregulation
Pressure-flow autoregulation involves myogenic responses of vascular
smooth muscle to stretching and acts to keep local blood flow constant, despite changes
in systemic arterial pressure. Within limits, an increase in transmural pressure
raises myogenic tone, causes vasoconstriction, and prevents hypertension-induced
elevations of local blood flow. Conversely, a decrease in transmural pressure lowers
myogenic tone, causing vasodilation, which helps preserve organ perfusion during
systemic hypotension.
Pressure-flow autoregulation of the hepatic artery is present
to a certain extent in metabolically active liver (postprandial) but is usually absent
in the fasted state.[14]
Because pressure-flow
autoregulation does not exist in the portal circulation, decreases in systemic blood
pressure beget proportional decreases in portal venous blood flow.[15]
[16]
Thus, pressure-flow autoregulation is unlikely
to have an important influence on hepatic blood flow intraoperatively, with the possible
exception of emergency procedures performed on patients in the fed state.
Metabolic Control
Constituents of blood can influence hepatic arterial and portal
venous blood flow.[17]
Decreases in the pH or oxygen
tension of the portal blood are often associated with increases in hepatic arterial
flow. Postprandial hyperosmolarity increases both the hepatic arterial and the portal
venous flow.[17]
Changes in metabolic or respiratory
status, such as hypercarbia, alkalosis, or arterial hypoxemia, can also influence
liver blood flow.
Hepatic Arterial Buffer Response
The hepatic arterial buffer response acts to ensure that changes
in portal venous flow induce reciprocal changes in hepatic arterial flow.[18]
This reciprocal relation helps
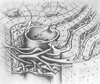 Figure 19-4
Relationship of branches of the portal vein (PV), hepatic
artery (HA), and bile duct (BD). Notice the peribiliary capillary plexus that envelops
the bile ducts. These three structures constitute a portal triad, which is a transverse
section of a portal canal. (Reprinted with permission from Jones AL: Anatomy
of the normal liver. In Zakim D, Boyer T [eds]:
Hepatology: A Textbook of Liver Disease, 3rd ed. Philadelphia, WB Saunders, 1996,
p 3.)
Figure 19-4
Relationship of branches of the portal vein (PV), hepatic
artery (HA), and bile duct (BD). Notice the peribiliary capillary plexus that envelops
the bile ducts. These three structures constitute a portal triad, which is a transverse
section of a portal canal. (Reprinted with permission from Jones AL: Anatomy
of the normal liver. In Zakim D, Boyer T [eds]:
Hepatology: A Textbook of Liver Disease, 3rd ed. Philadelphia, WB Saunders, 1996,
p 3.)
balance hepatic needs for oxygen and blood flow. The buffer response works through
the synthesis and washout of adenosine (i.e., a vasodilator) from the periportal
region.[19]
As portal venous flow decreases, adenosine
builds up in the periportal region; increases in periportal adenosine cause arteriolar
resistance to fall and hepatic
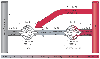 Figure 19-5
Adrenoceptor subtypes (α1
, α2
,
β2
) and intravascular pressures throughout the splanchnic circulation.
Splanchnic arteries represent all arterial vessels
of the pre-portal organs; splanchnic veins represent
the pooled venous blood from all these organs. (Redrawn with permission
from Gelman S, Mushlin PS: Catecholamine induced changes in the splanchnic circulation
affecting systemic hemodynamics. Anesthesiology 100:434–439, 2004.)
Figure 19-5
Adrenoceptor subtypes (α1
, α2
,
β2
) and intravascular pressures throughout the splanchnic circulation.
Splanchnic arteries represent all arterial vessels
of the pre-portal organs; splanchnic veins represent
the pooled venous blood from all these organs. (Redrawn with permission
from Gelman S, Mushlin PS: Catecholamine induced changes in the splanchnic circulation
affecting systemic hemodynamics. Anesthesiology 100:434–439, 2004.)
arterial flow to rise. Conversely, an increase in portal venous flow washes out
adenosine from the periportal region, which raises arteriolar resistance and lowers
hepatic arterial flow. Neural, myogenic, or metabolic influences (e.g., portal venous
oxygen content or pH) may alter the buffer response.[20]
Although the buffer response can substantially increase hepatic arterial flow, it
cannot preserve total hepatic blood flow when portal venous flow falls precipitously.
Furthermore, pathophysiologic states, such as endotoxemia and splanchnic hypoperfusion,
may decrease or even abolish the buffer response.[21]
[22]
 Figure 19-4
Relationship of branches of the portal vein (PV), hepatic
artery (HA), and bile duct (BD). Notice the peribiliary capillary plexus that envelops
the bile ducts. These three structures constitute a portal triad, which is a transverse
section of a portal canal. (Reprinted with permission from Jones AL: Anatomy
of the normal liver. In Zakim D, Boyer T [eds]:
Hepatology: A Textbook of Liver Disease, 3rd ed. Philadelphia, WB Saunders, 1996,
p 3.)
Figure 19-4
Relationship of branches of the portal vein (PV), hepatic
artery (HA), and bile duct (BD). Notice the peribiliary capillary plexus that envelops
the bile ducts. These three structures constitute a portal triad, which is a transverse
section of a portal canal. (Reprinted with permission from Jones AL: Anatomy
of the normal liver. In Zakim D, Boyer T [eds]:
Hepatology: A Textbook of Liver Disease, 3rd ed. Philadelphia, WB Saunders, 1996,
p 3.) Figure 19-5
Adrenoceptor subtypes (α1
, α2
,
β2
) and intravascular pressures throughout the splanchnic circulation.
Splanchnic arteries represent all arterial vessels
of the pre-portal organs; splanchnic veins represent
the pooled venous blood from all these organs. (Redrawn with permission
from Gelman S, Mushlin PS: Catecholamine induced changes in the splanchnic circulation
affecting systemic hemodynamics. Anesthesiology 100:434–439, 2004.)
Figure 19-5
Adrenoceptor subtypes (α1
, α2
,
β2
) and intravascular pressures throughout the splanchnic circulation.
Splanchnic arteries represent all arterial vessels
of the pre-portal organs; splanchnic veins represent
the pooled venous blood from all these organs. (Redrawn with permission
from Gelman S, Mushlin PS: Catecholamine induced changes in the splanchnic circulation
affecting systemic hemodynamics. Anesthesiology 100:434–439, 2004.)