FLUMAZENIL
Flumazenil (Anexate, Romazicon) is the first benzodiazepine antagonist
approved for clinical use.[388]
Preclinical pharmacologic
studies with flumazenil revealed it to be a benzodiazepine receptor ligand with high
affinity, great specificity, and minimal intrinsic effect by definition.[377]
Flumazenil, like the agonists that it replaces at the benzodiazepine receptor, interacts
with the receptor in a concentration-dependent manner. Because it is a competitive
antagonist at the benzodiazepine receptor, its antagonism is reversible and surmountable.
Flumazenil has minimal intrinsic activity,[377]
[417]
which means that its benzodiazepine receptor
agonist effects are very weak, significantly less than those of clinical agonists.
Flumazenil, like all competitive antagonists at receptors, does not displace the
agonist but rather occupies the receptor when an agonist dissociates from the receptor.
The half-time (or half-life) of a receptor-ligand bond is a few milliseconds to
a few seconds, and new ligand-receptor bonds are then immediately formed. This dynamic
situation accounts for the ability of either an agonist or an antagonist to readily
occupy the receptor. The proportion of receptors occupied by the agonist in the
presence of an antagonist obeys the law of mass action and depends on the affinities
and concentrations of the two ligands.[372]
[377]
Equation 1 expresses this relationship[418]
:
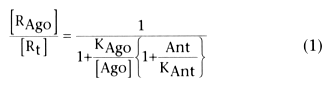
where [RAgo
] is the receptor concentration of agonist, [Rt
]
is the total number of receptors, KAgo
is the dissociation constant for
agonist, KAnt
is the dissociation constant for antagonist, [Ago] is the
concentration of agonist at the receptor, and [Ant] is the concentration of antagonist
at the receptor.
The ratio of agonist to total receptors produces the effects of
the agonist drug, but an antagonist can alter this ratio, depending on its concentration
and dissociation constant (see Equation 1). Thus, flumazenil, which is an avid (high-affinity)
ligand, will replace a relatively weak agonist such as diazepam as long as it is
given in sufficient dose (i.e., high [Ant]). However, flumazenil is cleared relatively
rapidly, and the net result is that [Ant] is reduced over time as compared with [Ago];
thus, the proportion of receptors occupied by agonist will increase, and the potential
for resedation exists ( Fig. 10-19
).
This situation is less likely to occur when flumazenil is used to reverse midazolam,
which has more rapid clearance than other
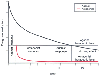 Figure 10-19
Schematic representation of the interaction of a short-acting
antagonist with a longer-acting agonist resulting in resedation. The upper
curve shows disappearance of agonist from blood, and the lower
curve shows disappearance of antagonist from plasma. Four conditions
are represented: I, agonist response; II, antagonist response (the antagonist reverses
the agonist effect); III, agonist response (resedation or resumption of agonist response
with disappearance of short-lasting antagonist); and IV, no drug effect, with disappearance
of both agonist and antagonist (both drugs are below the therapeutic level).
Figure 10-19
Schematic representation of the interaction of a short-acting
antagonist with a longer-acting agonist resulting in resedation. The upper
curve shows disappearance of agonist from blood, and the lower
curve shows disappearance of antagonist from plasma. Four conditions
are represented: I, agonist response; II, antagonist response (the antagonist reverses
the agonist effect); III, agonist response (resedation or resumption of agonist response
with disappearance of short-lasting antagonist); and IV, no drug effect, with disappearance
of both agonist and antagonist (both drugs are below the therapeutic level).
benzodiazepine agonists do.[419]
Another important
finding is that in the presence of extremely high doses of agonist (e.g., when a
mistake in dosing has occurred or suicide is attempted), flumazenil in low dose attenuates
the deep CNS depression (loss of consciousness, respiratory depression) by reducing
the fractional receptor occupancy by the agonist without decreasing the agonist effects
that occur at low fractional receptor occupancy (drowsiness, amnesia). Conversely,
high doses of flumazenil in the presence of low doses of agonist completely reverse
virtually all the agonist effects. Flumazenil can precipitate withdrawal symptoms
in animals or in humans physically dependent on a benzodiazepine receptor agonist.
[420]
Such symptoms are not a problem when flumazenil
is used to reverse benzodiazepine receptor agonists in anesthesia.
Physicochemical Characteristics
Flumazenil, synthesized in 1979, is similar to midazolam and other
classic benzodiazepines except for absence of the phenyl group, which is replaced
by a carbonyl group (see Fig. 10-10
).
[421]
It forms a colorless, crystalline powder,
has a dissociation constant of 1.7, and has weak but sufficient water solubility
to permit its preparation in aqueous solution. Its octanol/aqueous buffer partition
coefficient is 14, and it demonstrates moderate lipid solubility at pH 7.4.[421]

 Figure 10-19
Schematic representation of the interaction of a short-acting
antagonist with a longer-acting agonist resulting in resedation. The upper
curve shows disappearance of agonist from blood, and the lower
curve shows disappearance of antagonist from plasma. Four conditions
are represented: I, agonist response; II, antagonist response (the antagonist reverses
the agonist effect); III, agonist response (resedation or resumption of agonist response
with disappearance of short-lasting antagonist); and IV, no drug effect, with disappearance
of both agonist and antagonist (both drugs are below the therapeutic level).
Figure 10-19
Schematic representation of the interaction of a short-acting
antagonist with a longer-acting agonist resulting in resedation. The upper
curve shows disappearance of agonist from blood, and the lower
curve shows disappearance of antagonist from plasma. Four conditions
are represented: I, agonist response; II, antagonist response (the antagonist reverses
the agonist effect); III, agonist response (resedation or resumption of agonist response
with disappearance of short-lasting antagonist); and IV, no drug effect, with disappearance
of both agonist and antagonist (both drugs are below the therapeutic level).