Physicochemical Characteristics
Chemistry and Formulation
Barbiturates are hypnotically active drugs that are derivatives
of barbituric acid (2,4,6-trioxohexahydropyrimidine), a hypnotically inactive pyrimidine
nucleus formed by the condensation of malonic acid and urea ( Fig.
10-7
). The two major divisions of barbiturates are those with an oxygen
at position 2 (oxybarbiturates) and those with a sulfur at position 2 (thiobarbiturates).
Through keto-enol tautomerization, the oxygen or sulfur at position 2 becomes a
reactive species in the enol form that allows for the formation of water-soluble
barbiturate salts in alkaline solutions. This solubility permits the intravenous
use of barbiturates. Although tautomerization to the enol form allows for the creation
of salts, it is substitution of the hydrogen attached to the carbon atom in position
5 by aryl or alkyl groups that gives the barbiturates their hypnotic activity. A
list of hypnotically active barbiturates can be found in Table
10-4
. From this list, only the thiobarbiturates thiopental and thiamylal
and the oxybarbiturate methohexital are commonly used for induction of anesthesia
( Fig. 10-8
).
The formulation of barbiturates involves preparation of them as
sodium salts (mixed with 6% anhydrous sodium carbonate by weight) and then reconstitution
with either water or normal saline to produce a 2.5% solution of thiopental, a 2.0%
solution of thiamylal, or a 1.0% solution of methohexital. Thiobarbiturates are
stable for 1 week if refrigerated after reconstitution, and methohexital remains
available for use for up to 6 weeks after reconstitution. A decrease in alkalinity
of the solution can result in precipitation of barbiturates as free
*Only
the ultrashort-acting drugs are commonly used for induction.
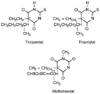 Figure 10-8
Hypnotically active barbiturates commonly used for induction
with their asymmetric centers indicated by an asterisk.
Figure 10-8
Hypnotically active barbiturates commonly used for induction
with their asymmetric centers indicated by an asterisk.
acids, which is why they cannot be reconstituted with lactated Ringer's solution
or mixed with other acidic solutions. Examples of drugs that are not to be coadministered
or mixed in solution with barbiturates are pancuronium, vecuronium, atracurium, alfentanil,
sufentanil, and midazolam. Studies have shown that in rapid-sequence induction,
mixing of thiopental with vecuronium or pancuronium results in the formation of a
precipitate that may occlude the intravenous line.[289]
Structure-Activity Relationships
Substitutions at the 5, 2, and 1 positions confer different pharmacologic
activities to the barbiturate nucleus. Substitutions at position 5 with either aryl
or alkyl groups produce hypnotic and sedative effects. A phenyl group substitution
at C5 produces anticonvulsant activity. An increase in the length of one or both
side chains of an alkyl group at C5 increases hypnotic potency. The barbiturates
used in clinical practice have either an oxygen or sulfur at C2. Substitution of
sulfur at position 2 produces a more rapid onset of action. The addition of a methyl
or ethyl group at position 1 may also produce a more rapid onset of action, but excitatory
side effects, including tremor, hypertonus, and involuntary movement, may occur with
administration. A comparison of the potency, onset of action, length of action,
and side effects is presented in Table
10-5
.
Stereoisomerism
It has been known since the 1970s that optical isomers of barbiturates
can have different anesthetic activities in mammals.[290]
Several of the barbiturates, including thiopental, thiamylal, methohexital, secobarbital,
and pentobarbital, have asymmetric carbon atoms in at least one of the side chains
attached to carbon 5 of the barbiturate ring. The S (l)
isomers of these barbiturates are roughly twice as potent as the R (d)
isomers despite their similar access to the CNS.[290]
[291]
[292]
[293]
[294]
The fundamental importance of this fact is
that stereoisomers of the same drug
TABLE 10-5 -- Relationship of chemical grouping to clinical action and potency of barbiturates
|
Substituents |
|
|
Group |
Position 1 |
Position 2 |
Group Characteristics When Given IV |
|
Oxybarbiturates |
H |
O |
Delay in onset of action, the degree of which is dependent on
the 5 and 5' side chain. Useful as a basal hypnotic. Prolonged action. Excitatory
side effects |
|
Methylated thiobarbiturates |
CH3
|
O |
Usually rapid acting with fairly rapid recovery. High incidence
of excitatory side effects |
|
Thiobarbiturates |
H |
S |
Rapid acting, usually smooth onset of sleep, fairly prompt recovery |
|
Methylated thiobarbiturates |
CH3
|
S |
Rapid onset of action and very rapid recovery, but too many excitatory
side effects to make clinical use feasible |
|
Modified from Dundee JW, Wyant GM: Intravenous Anaesthesia.
Edinburgh, Churchill Livingstone, 1974. |
can have different CNS potency and activity, which is suggestive of the existence
of chirally active centers on barbiturate receptors rather than nonspecific actions
mediated by the drug. For example, the racemic mixture of pentobarbital has isomers
that produce different actions in cultured mammalian neurons, with the (+) isomers
being primarily excitatory and the (-) isomers being primarily inhibitory. Thus,
the overall CNS activity of a particular barbiturate depends on the summation of
the effects of the stereoisomers at each point of action and the relative potency
of the action at each site. Similarly, the duration of action for any given barbiturate
is dependent on the summation of the duration of effects of each isomer at its receptor
site.[291]
 Figure 10-8
Hypnotically active barbiturates commonly used for induction
with their asymmetric centers indicated by an asterisk.
Figure 10-8
Hypnotically active barbiturates commonly used for induction
with their asymmetric centers indicated by an asterisk.