BARBITURATES
History
Barbituric acid, a combination of urea and malonic acid that is
lacking in sedative properties, was first synthesized in 1864 by J.F.W. Adolph von
Baeyer, a Nobel Prize-winning organic chemist.[278]
Barbital (diethylbarbituric acid), the first barbiturate with sedative properties,
was reported by Fischer and von Mering in 1903.[279]
This oral hypnotic was very long acting and very popular as a sedative in clinical
practice. However, it was not until 1920 with the introduction of Somnifen, a mixture
of the barbiturate
salts of diethylbarbituratic and diallylbarbituratic acids, that intravenous barbiturates
became widely available for clinical use. Somnifen was described by Redonnet in
1920 and was first used in clinical practice by Bardet and Bardet in 1921 on a labor
and delivery ward. It was first used in surgical practice in 1924 by Fredet and
Perlis, and its use continued for many years in France and Germany.[280]
[281]
The first ultrashort-acting barbiturate, hexobarbital, was prepared
by Kropp and Taub and was introduced into clinical use in July 1932 by H. Weese and
W. Scharpff.[282]
Although hexobarbital was used
widely in Europe, it did not enjoy the same level of success in North America. In
1929, the use of amobarbital (Amytal) was reported by Zerfas and colleagues, and
it quickly became the most common intravenous anesthetic in North America.[283]
The thiobarbiturates were first described in 1903. However, because
of fatal experiments in dogs, their use
 Figure 10-6
Reproduction of the first administration of thiopental
by Waters on March 8, 1934. (From Dundee JW, Wyant GM: Intravenous Anaesthesia.
Edinburgh, Churchill Livingstone, 1974.)
Figure 10-6
Reproduction of the first administration of thiopental
by Waters on March 8, 1934. (From Dundee JW, Wyant GM: Intravenous Anaesthesia.
Edinburgh, Churchill Livingstone, 1974.)
was not further explored until the 1930s.[283]
In 1935, Tabern and Volwiler synthesized a series of sulfur-containing barbiturates,
of which thiopental became the most widely used.[284]
Thiopental was introduced clinically by Ralph Waters and John Lundy and became preferred
clinically because of its rapid onset of action and short duration, without the excitatory
effects of hexobarbital ( Fig. 10-6
).
[283]
[285]
Though criticized after many casualties during the attack on Pearl
Harbor as "the ideal form of euthanasia in war surgery," barbiturates continued to
be widely used in clinical practice.[286]
Even
though many other barbiturate derivatives has been synthesized throughout the past
several decades, none has enjoyed the clinical success and popularity of thiopental.
Thiopental has survived the test of time as an intravenous anesthetic drug. A more
detailed description of the history of the barbiturates in anesthesia is available
elsewhere.[278]
[287]
[288]
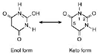 Figure 10-7
The keto and enol tautomeric forms of barbituric acid
with the sites of substitution in the hypnotically active barbiturates identified
as 1, 2, and 5.
Figure 10-7
The keto and enol tautomeric forms of barbituric acid
with the sites of substitution in the hypnotically active barbiturates identified
as 1, 2, and 5.
 Figure 10-6
Reproduction of the first administration of thiopental
by Waters on March 8, 1934. (From Dundee JW, Wyant GM: Intravenous Anaesthesia.
Edinburgh, Churchill Livingstone, 1974.)
Figure 10-6
Reproduction of the first administration of thiopental
by Waters on March 8, 1934. (From Dundee JW, Wyant GM: Intravenous Anaesthesia.
Edinburgh, Churchill Livingstone, 1974.) Figure 10-7
The keto and enol tautomeric forms of barbituric acid
with the sites of substitution in the hypnotically active barbiturates identified
as 1, 2, and 5.
Figure 10-7
The keto and enol tautomeric forms of barbituric acid
with the sites of substitution in the hypnotically active barbiturates identified
as 1, 2, and 5.