Drager Narkomed 6000 and Fabius GS
Several important differences exist between the traditional circle
breathing systems of the newest Dräger products (see Fig.
9-2
). At first glance, the most notable difference lies in the appearance
and design of the ventilators used with these systems. From the inconspicuous, horizontally
mounted, Divan piston ventilator of the Narkomed 6000 (see Fig.
9-2
) to the vertically mounted and visible piston ventilator of the Fabius
GS with its absent flow tubes and glowing electronic fresh gas flow indicators, these
systems appear drastically different from traditional anesthesia systems. The piston
ventilator of the Dräger Narkomed 6000, Dräger Fabius GS, and Dräger
Primus anesthesia systems may be classified as electrically powered, piston driven,
single circuit, and electronically controlled with fresh gas decoupling.[20]
[22]
[23]
The circle breathing systems used by these workstations incorporate
a feature known as fresh gas decoupling (FGD). Incorporation
of this technology to enhance safety for the patient has required a significant redesign
of the traditional circle system. A functional schematic of a circle system similar
to the one used by the Dräger Narkomed 6000 during the inspiratory and expiratory
phases of mechanical ventilation can be seen in Figure
9-26
. To understand the operating principles of FGD, it is important to
have a good understanding of gas flows in a traditional circle system during the
inspiratory and expiratory phases of mechanical ventilation. A complete discussion
of this was presented earlier in "Operating Principles of Ascending Bellows Ventilators."
The key concept of the fresh gas-decoupled breathing system can
be illustrated during the inspiratory phase of mechanical ventilation. With the
traditional circle system, several events are occurring (see Fig.
9-24A
): continuous fresh gas flows from the flow meters or the oxygen
flush valve, or both, and enters the circle system at the fresh gas inlet; the ventilator
is delivering the prescribed tidal volume to the patient's lungs; and the ventilator's
relief valve (i.e., ventilator's exhaust valve) is closed, and no gas is escaping
the circle system except into the patient's lungs.[150]
In a traditional circle system, when these events coincide and fresh gas inflow
is coupled directly into the circle system, the total volume delivered to the patient's
lungs is the sum of the volume from the ventilator plus the volume of gas that enters
the circle through the common gas inlet. In contrast, when FGD is used, during the
inspiratory phase (see Fig. 9-26A
),
the fresh gas coming from the anesthesia machine is diverted into the reservoir bag
by a decoupling valve that is between the fresh gas source and the ventilator's circuit.
The reservoir (breathing) bag serves as an accumulator for fresh gas until the expiratory
phase begins. During the expiratory phase (see Fig.
9-26B
), the decoupling valve opens, allowing the accumulated fresh gas
in the reservoir bag to be drawn into the circle system to refill the piston ventilator's
chamber or descending bellows. Because the ventilator's exhaust valve also opens
during the expiratory phase, excess fresh gas and gases exhaled by the patient are
allowed to escape to the scavenging system.
Current fresh gas-decoupled systems are designed with piston-type
or descending bellows-type ventilators. Because the bellows in either of these systems
refill under slight negative pressure, it allows the accumulated fresh gas from the
reservoir bag to be drawn into the ventilator for delivery to the patient during
the next cycle of the ventilator. Because of this design requirement, it is unlikely
that FGD can be used with conventional ascending bellows ventilators, which refill
under slight positive pressure.
The most significant advantage of circle systems using FGD is
a decreased risk of barotrauma. With a traditional circle system, increases in fresh
gas flow from the flow meters or from inappropriate use of the oxygen flush valve
may contribute directly to the tidal volume, which if excessive may result in pneumothorax.
Because systems with FGD isolate fresh gas from coming into the system from the
patient while the ventilator's exhaust valve is closed, the risk of barotrauma is
greatly reduced.
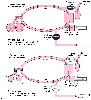 Figure 9-26
Inspiratory (A) and expiratory
(B) phases of gas flow in a Dräger-type circle
system with a piston ventilator and fresh gas decoupling. NPR valve, negative-pressure
relief valve. (Adapted from Brockwell RC: New Circle System Designs: A
Collection of 12 Illustrations. Copyright 2003.)
Figure 9-26
Inspiratory (A) and expiratory
(B) phases of gas flow in a Dräger-type circle
system with a piston ventilator and fresh gas decoupling. NPR valve, negative-pressure
relief valve. (Adapted from Brockwell RC: New Circle System Designs: A
Collection of 12 Illustrations. Copyright 2003.)
Possibly the greatest disadvantage to the new anesthesia circle
systems that use FGD is the possibility of entraining room air into the patient's
gas circuit. The bellows or piston of a circle system with FGD refills under slight
negative pressure. If the reservoir bag volume plus the returning volume of gas
exhaled from the patient's lungs is inadequate to refill the bellows or piston, negative
airway pressures may develop in the patient. To avoid development of negative airway
pressure, a negative-pressure relief valve is placed in the breathing system (see
Fig. 9-26
). If breathing
system pressure falls below a preset value, such as -2 cm H2
O, the relief
valve opens, and room air is entrained into the patient's gas circuit. If this goes
undetected, the entrained atmospheric gases can lead to dilution of both the inhaled
anesthetic agents and an enriched oxygen mixture, lowering an enriched oxygen concentration
toward 21%.[20]
Unchecked, this could lead to intraoperative
awareness or hypoxia. High-priority alarms with audio and visual alerts should notify
the user that fresh gas flow is inadequate and that room air is being entrained.
Another potential problem with the Dräger-Narkomed 6000-type
FGD system lies in its reliance on the reservoir bag to accumulate the incoming fresh
gas. If the reservoir bag is removed from the machine during mechanical ventilation
or it has a significant leak from poor fit on the bag mount or a perforation, room
air may enter the breathing circuit as the ventilator refills during the expiratory
phase. This may also result in dilution of both the inhaled anesthetic agents or
an enriched oxygen mixture, potentially resulting in awareness of the patient during
anesthesia or hypoxia. This type of a disruption can lead to significant pollution
of the operating room with nitrous oxide or inhaled anesthetic agents as fresh gases
escape into the atmosphere.
 Figure 9-26
Inspiratory (A) and expiratory
(B) phases of gas flow in a Dräger-type circle
system with a piston ventilator and fresh gas decoupling. NPR valve, negative-pressure
relief valve. (Adapted from Brockwell RC: New Circle System Designs: A
Collection of 12 Illustrations. Copyright 2003.)
Figure 9-26
Inspiratory (A) and expiratory
(B) phases of gas flow in a Dräger-type circle
system with a piston ventilator and fresh gas decoupling. NPR valve, negative-pressure
relief valve. (Adapted from Brockwell RC: New Circle System Designs: A
Collection of 12 Illustrations. Copyright 2003.)