|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Controlled vaporization of desflurane requires an electrically heated, pressurized vaporizer because of desflurane's unique physical properties. [89] [90] The vapor pressure of desflurane is three to four times that of contemporary inhaled anesthetics, and it boils at 22.8°C,[91] which is near room temperature (see Fig. 9-13 ). Desflurane has a minimum alveolar anesthetic concentration (MAC) of 6% to 7%.[91] Desflurane is valuable because it has a low blood gas solubility coefficient of 0.45 at 37°C, and recovery from anesthesia is more rapid than with many other potent inhaled anesthetics.[91]
Desflurane's high volatility and moderate potency preclude its use with contemporary variable-bypass vaporizers such as Datex-Ohmeda Tec 4, Tec 5, or Tec 7 and the North American Dräger Vapor 19.n or 20.n for two reasons. [89] First, the vapor pressure of desflurane is near 1 atm. The vapor pressures of enflurane, isoflurane, halothane, and desflurane at 20°C are 172, 240, 244, and 669 mm Hg,[91] respectively (see Fig. 9-13 ). Normal flow through a traditional vaporizer would vaporize many more volumes of desflurane. For example, at 1 atm and 20°C, 100 mL/min passing through the vaporizing chamber entrains 735 mL/min of desflurane versus 29, 46, and 47 mL/min of enflurane, isoflurane, and halothane, respectively.[89] Under these same conditions, the amount of bypass flow necessary to achieve sufficient distribution of anesthetic vapor to produce 1% desflurane output is approximately 73 L/min, compared with 5 L/min or less for the other three anesthetics. Above 22.8°C at 1 atm, desflurane boils. The amount of vapor produced is limited only by the heat energy available from the vaporizer because of its specific heat.[89]
A second reason is that contemporary vaporizers lack an external heat source. Although desflurane has a heat of vaporization approximately equal to that of enflurane, isoflurane, and halothane, its MAC is four to nine times higher than that of the other three inhaled anesthetics. The absolute amount of desflurane vaporized over a given period is considerably higher than with other anesthetics. Supplying desflurane in higher concentrations causes excessive cooling of the vaporizer. In the absence of an external heat source, temperature compensation using traditional mechanical devices would be almost impossible over a broad clinical range of temperatures because of desflurane's steep vapor pressure-versus-temperature curve (see Fig. 9-13 ).[89]
To achieve controlled vaporization of desflurane, Datex-Ohmeda introduced the Tec 6 vaporizer into widespread clinical practice in 1993. This was the first commercially available vaporizer to be electrically heated and pressurized. [92] The physical appearance and operation of the Tec 6 are similar to those of contemporary vaporizers, but some aspects of the internal design and operating principles are radically different. The Tec-6 Plus is a somewhat newer version of the original Tec-6. This device has the same basic Tec-6 design with an enhanced audio alarm system.
A simplified schematic drawing of the Tec 6 vaporizer is shown in Figure 9-18 . The vaporizer has two independent gas circuits arranged in parallel. The fresh gas circuit is shown in red, and the vapor circuit is shown in white. The fresh gas from the flow meters enters at the fresh gas inlet, passes through a fixed restrictor (R1), and exits at the vaporizer gas outlet. The vapor circuit originates
 Figure 9-18
Simplified schematic of the Tec 6 desflurane vaporizer.
The vaporizer has two independent gas circuits arranged in parallel. The fresh
gas circuit is shown in red, and the vapor circuit is shown in white. The fresh
gas from the flow meters enters at the fresh gas inlet, passes through a fixed restrictor
(R1), and exits at the vaporizer gas outlet. The vapor circuit originates at the
desflurane sump, which is electrically heated and thermostatically controlled to
39°C, a temperature well above desflurane's boiling point. The heated sump assembly
serves as a reservoir of desflurane vapor. Downstream from the sump is the shut-off
valve. After the vaporizer warms up, the shut-off valve fully opens when the concentration
control valve is turned to the on position. A pressure-regulating valve located
downstream from the shut-off valve downregulates the pressure. The operator controls
desflurane output by adjusting the concentration control valve (R2), which is a variable
restrictor. (Adapted from Andrews JJ: Operating Principles of the Ohmeda
Tec 6 Desflurane Vaporizer: A Collection of Twelve Color Illustrations. Washington,
DC, Library of Congress, 1996.)
Figure 9-18
Simplified schematic of the Tec 6 desflurane vaporizer.
The vaporizer has two independent gas circuits arranged in parallel. The fresh
gas circuit is shown in red, and the vapor circuit is shown in white. The fresh
gas from the flow meters enters at the fresh gas inlet, passes through a fixed restrictor
(R1), and exits at the vaporizer gas outlet. The vapor circuit originates at the
desflurane sump, which is electrically heated and thermostatically controlled to
39°C, a temperature well above desflurane's boiling point. The heated sump assembly
serves as a reservoir of desflurane vapor. Downstream from the sump is the shut-off
valve. After the vaporizer warms up, the shut-off valve fully opens when the concentration
control valve is turned to the on position. A pressure-regulating valve located
downstream from the shut-off valve downregulates the pressure. The operator controls
desflurane output by adjusting the concentration control valve (R2), which is a variable
restrictor. (Adapted from Andrews JJ: Operating Principles of the Ohmeda
Tec 6 Desflurane Vaporizer: A Collection of Twelve Color Illustrations. Washington,
DC, Library of Congress, 1996.)
The vapor flow through R2 joins the fresh gas flow through R1 at a point downstream from the restrictors. Until this point, the two circuits are physically divorced. They are interfaced pneumatically and electronically, however, through differential pressure transducers, a control electronics system, and a pressure-regulating valve. When a constant fresh gas flow rate encounters the fixed restrictor, R1, a specific backpressure, proportional to the fresh gas flow rate, pushes against the diaphragm of the control differential pressure transducer. The differential pressure transducer conveys the pressure difference between the fresh gas circuit and the vapor circuit to the control electronics system. The control electronics system regulates the pressure-regulating valve so that the pressure in the vapor circuit equals the pressure in the fresh gas circuit. This equalized pressure supplying R1 and R2 is the working pressure, and the working pressure is constant at a fixed fresh gas flow rate. If the operator increases the fresh gas flow rate, more backpressure is exerted on the diaphragm of the control pressure transducer, and the working pressure of the vaporizer increases.[89]
Table 9-1 shows the approximate correlation between fresh gas flow rate and working pressure for a typical vaporizer. At a fresh gas flow rate of 1 L/min, the working pressure is 10 millibars or 7.4 mm Hg gauge. At a fresh gas flow rate of 10 L/min, the working pressure is 100 millibars or 74 mm Hg gauge. There is a linear
|
|
Working Pressure at R1 and R2 by Different Gauges * | ||
|---|---|---|---|
| Fresh Gas Flow Rate (L/min) | Millibars | cm H2 O | mm Hg |
| 1 | 10 | 10.2 | 7.4 |
| 5 | 50 | 51.0 | 37.0 |
| 10 | 100 | 102.0 | 74.0 |
| R1, fixed restrictor; R2, concentration control valve. | |||
| Adapted from Andrews JJ, Johnston RV Jr: The new Tec 6 desflurane vaporizer. Anesth Analg 76:1338, 1993. | |||
Two examples are provided to demonstrate the operating principles of the Tec 6.[72] In the first, there is a constant fresh gas flow rate of 1 L/min with an increase in the dial setting. With a fresh gas flow rate of 1 L/min, the working pressure of the vaporizer is 7.4 mm Hg; the pressure supplying R1 and R2 is 7.4 mm Hg. As the operator increases the dial setting, the opening at R2 becomes larger, allowing more vapor to pass through R2. Specific vapor flow values at different dial settings are shown in Table 9-2 .
In the second example, there is a constant dial setting with an
increase in fresh gas flow from 1 to 10 L/min. At a fresh gas flow rate of 1 L/min,
the working pressure is 7.4 mm Hg, and at a dial setting of 6%, the vapor flow rate
through R2 is 64 mL/min (see Table
9-1
and Table 9-2
).
With a 10-fold increase in the fresh gas flow rate, there is a concomitant 10-fold
increase in the working pressure to 74 mm Hg. The ratio of resistances of R2 to
R1 is constant at a fixed dial setting of 6%. Because R2 is supplied by 10 times
more pressure, the vapor flow rate through R2 increases 10-fold to 640 mL/min. Vaporizer
output is
| Dial Setting (Vol %) * | Fresh Gas Flow Rate (L/min) | Approximate Vapor Flow Rate Through R2 (mL/min) |
|---|---|---|
| 1 | 1 | 10 |
| 6 | 1 | 64 |
| 12 | 1 | 136 |
| 18 | 1 | 220 |
| R2, concentration control valve. | ||
| Adapted from Andrews JJ, Johnston RV Jr: The new Tec 6 desflurane vaporizer. Anesth Analg 76:1338, 1993. | ||

Varied altitude and carrier gas composition influence Tec 6 output.
Unlike contemporary variable-bypass vaporizers, the Tec 6 vaporizer
requires manual adjustments of the concentration control dial at altitudes other
than sea level to maintain a constant partial pressure of anesthetic. The Tec 6
works at absolute pressures; therefore, altitude makes no difference to the vaporizer's
performance. It can accurately deliver the dialed volume percent of desflurane.
However, when this gas is brought to ambient atmospheric pressure at high altitudes,
the volume percent represents an absolute decrease in the partial pressure of the
anesthetic, unlike the contemporary variable-bypass vaporizers, which deliver a constant
partial pressure of anesthetic. To compensate for the reduction of partial pressure
of vapor at altitude, the Tec 6 rotary valve must be advanced to maintain the required
partial pressure of anesthetic. The required dial setting may be calculated using
the following formula[92]
:
Required dial setting = Normal dial setting (vol %) ×
760 mm Hg/Ambient pressure (mm Hg)
For example, at an altitude of 2000 m or 6564 feet, where the ambient pressure is
608 mm Hg, the operator must advance the concentration control dial from 10% to 12.5%
to maintain the required anesthetic partial pressure.[92]
In hyperbaric settings, the operator must decrease the dial setting to prevent delivery
of an overdose.
Vaporizer output approximates the dial setting when oxygen is the carrier gas because the Tec 6 vaporizer is calibrated using 100% oxygen.[92] At low flow rates when a carrier gas other than 100% oxygen is used, a clear trend toward reduction in vaporizer output emerges. This reduction parallels the proportional decrease in viscosity of the carrier gas. Nitrous oxide has a lower viscosity than oxygen, and the backpressure generated by resistor R1 (see Fig. 9-18 ) is less when nitrous oxide is the carrier gas, and the working pressure is reduced. At low flow rates using nitrous oxide as the carrier gas, vaporizer output is approximately 20% less than the dial setting. This suggests that, at clinically useful fresh gas flow rates, the gas flow across resistor R1 is laminar, and the working pressure is proportional to both the fresh gas flow rate and the viscosity of the carrier gas.[94]
Because desflurane's vapor pressure is near 1 atm, misfilling contemporary vaporizers with desflurane can theoretically cause desflurane overdose and hypoxemia.[95] Datex-Ohmeda has introduced a unique, anesthetic-specific filling system to minimize occurrence of this hazard. The agent-specific filler of the desflurane bottle,
Major vaporizer faults cause the shut-off valve located just downstream from the desflurane sump (see Fig. 9-18 ) to close, producing a no-output situation. The valve is closed and a no-output alarm is activated immediately if any of the following conditions occur: the anesthetic level decreases to below 20 mL; the vaporizer is tilted; a power failure occurs; or there is a disparity between the pressure in the vapor circuit versus the pressure in the fresh gas circuit exceeding a specified tolerance.[92]
The Tec 6 vaporizer is an electrically heated, thermostatically controlled, constant-temperature, pressurized, electromechanically coupled, dual-circuit, gas-vapor blender. The pressure in the vapor circuit is electronically regulated to equal the pressure in the fresh gas circuit. At a constant fresh gas flow rate, the operator regulates vapor flow using a conventional concentration control dial. When the fresh gas flow rate increases, the working pressure increases proportionally. At a specific dial setting at different fresh gas flow rates, vaporizer output is
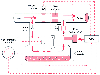 Figure 9-19
Simplified schematic of Datex-Ohmeda Aladin Cassette
Vaporizer. The arrows represent flow from the flow
meters, and the open circles represent anesthetic
vapor. The heart of the vaporizer is the electronically controlled flow control
valve located in the outlet of the vaporizing chamber. CPU, central processing unit,
FBC
, flow-measurement unit that measures flow through the bypass chamber,
FVC
, flow-measurement unit that measures flow through the vaporizing chamber,
P, pressure sensor, T, temperature sensor. (Modified from Andrews, JJ:
Operating Principles of the Datex-Ohmeda Aladin Cassette Vaporizer: A Collection
of Color Illustrations. Washington, DC, Library of Congress, 2000.)
Figure 9-19
Simplified schematic of Datex-Ohmeda Aladin Cassette
Vaporizer. The arrows represent flow from the flow
meters, and the open circles represent anesthetic
vapor. The heart of the vaporizer is the electronically controlled flow control
valve located in the outlet of the vaporizing chamber. CPU, central processing unit,
FBC
, flow-measurement unit that measures flow through the bypass chamber,
FVC
, flow-measurement unit that measures flow through the vaporizing chamber,
P, pressure sensor, T, temperature sensor. (Modified from Andrews, JJ:
Operating Principles of the Datex-Ohmeda Aladin Cassette Vaporizer: A Collection
of Color Illustrations. Washington, DC, Library of Congress, 2000.)
|
|
|
|
|
|
|
|
|
|
|
|
|