|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The blood supply to most of the eye is provided by the ophthalmic artery (OA), the first intracranial branch of the internal carotid artery. The intraorbital ON, retina, and choroid are supplied by branches of the OA, specifically, the posterior ciliary arteries (PCAs), central retinal artery (CRA), and the pial vessels along the ON ( Fig. 82-2 ). Anastomoses may be present between the internal carotid system from which the OA arises and the external carotid artery. The lacrimal artery anastomoses to the middle meningeal artery. In the eyelids and nose, distal branches of the OA and the external carotid artery anastomose.[8] Under normal conditions, these connections may not be significant in the ocular blood supply, but they may become potential pathways for iatrogenic particle embolization after intra-arterial injection into the external carotid system,[9] or conversely, they could compensate for obstruction in the OA.
The ocular branches of the OA are the CRA and PCA trunks, which branch into the main PCAs. Typically two to three PCA trunks branch into the medial and lateral PCAs ( Fig. 82-3 and Fig. 82-4 ). The main PCAs divide into short PCAs (sPCAs).[10] The sPCAs travel anteriorly and enter the sclera near the ON, usually in the nasal and temporal areas. sPCAs supply the posterior choroid and the anterior portion of the ON. The medial and lateral sPCAs form the
 Figure 82-2
The ophthalmic artery branches into the posterior ciliary
arteries and the central retinal artery. The central retinal artery enters the optic
nerve behind the globe and sends branches to supply the optic nerve. (From
Federman JL, Grouas P, Schubert H, et al [eds]: Retina and Vitreous, Textbook of
Ophthalmology. St Louis, CV Mosby, 1994.)
Figure 82-2
The ophthalmic artery branches into the posterior ciliary
arteries and the central retinal artery. The central retinal artery enters the optic
nerve behind the globe and sends branches to supply the optic nerve. (From
Federman JL, Grouas P, Schubert H, et al [eds]: Retina and Vitreous, Textbook of
Ophthalmology. St Louis, CV Mosby, 1994.)
Anatomic variation in the PCA circulation has important implications in the etiology of ischemic optic neuropathy (ION). The OA gives rise to one to five PCAs. One PCA is found in 3% of subjects, two in 48%, three in 39%, four in 8%, and five in 2%.[12] The PCAs enter the eye medial or lateral to the eyeball. One medial PCA is found in 71% of subjects and two in 29%; one lateral PCA is present in 75% of subjects, two in 20%, three in 2%, and none in 3%. Superior PCAs are present in 9% of subjects. Up to 20 sPCAs can be present and are subdivided into paraoptic and distal sPCAs. Paraoptic sPCAs enter the eye closest to the ON and supply the ON head (ONH). The distal sPCAs mostly supply the choroid.
The area supplied by the PCAs is subject to interindividual and even intraindividual variation. The distribution pattern can be extremely variable. The medial PCA may supply the entire nasal choroid and the ONH or, in some cases, provide no supply. The lateral PCA supplies the
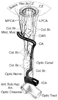 Figure 82-3
The origin, course, and branches of the ophthalmic artery,
including the posterior ciliary arteries, as seen from above. CAR, central retinal
artery; Col Br, collateral branches; CZ, circle of Zinn and Haller; ICA, internal
carotid artery; LPCA, lateral posterior ciliary artery; MPCA, medial posterior ciliary
artery; OA, ophthalmic artery. (From Pillanut LE, Harris A, Anderson DR,
Greve EL [eds]: Current Concepts on Ocular Blood Flow in Glaucoma. The Hague, Netherlands,
Kugler, 1999.)
Figure 82-3
The origin, course, and branches of the ophthalmic artery,
including the posterior ciliary arteries, as seen from above. CAR, central retinal
artery; Col Br, collateral branches; CZ, circle of Zinn and Haller; ICA, internal
carotid artery; LPCA, lateral posterior ciliary artery; MPCA, medial posterior ciliary
artery; OA, ophthalmic artery. (From Pillanut LE, Harris A, Anderson DR,
Greve EL [eds]: Current Concepts on Ocular Blood Flow in Glaucoma. The Hague, Netherlands,
Kugler, 1999.)
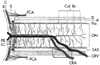 Figure 82-4
The blood supply to the optic nerve is illustrated.
The anterior portion of the optic nerve is located to the left,
whereas the posterior portion (closer to the brain) is on the right.
The anterior portion of the nerve derives its blood supply from the posterior ciliary
arteries (PCA) and the choroid (C), whereas the posterior optic nerve derives its
blood supply from penetrating pial arteries (Col br) and branches of the central
retinal artery (CRA). A, arachnoid; C, choroid; CRV, central retinal vein; D, dura;
LC, long ciliary artery; ON, optic nerve; PR, short posterior ciliary artery; R,
retina; S, sclera; SAS, subarachnoid space. (From Hayreh SS: Ischemic optic
neuropathy, University of lowa, Department of Opthalmology Internet Site. http://webeye.ophth.uiowa.edu/dept/aion/ion_fg2.jpg.)
Figure 82-4
The blood supply to the optic nerve is illustrated.
The anterior portion of the optic nerve is located to the left,
whereas the posterior portion (closer to the brain) is on the right.
The anterior portion of the nerve derives its blood supply from the posterior ciliary
arteries (PCA) and the choroid (C), whereas the posterior optic nerve derives its
blood supply from penetrating pial arteries (Col br) and branches of the central
retinal artery (CRA). A, arachnoid; C, choroid; CRV, central retinal vein; D, dura;
LC, long ciliary artery; ON, optic nerve; PR, short posterior ciliary artery; R,
retina; S, sclera; SAS, subarachnoid space. (From Hayreh SS: Ischemic optic
neuropathy, University of lowa, Department of Opthalmology Internet Site. http://webeye.ophth.uiowa.edu/dept/aion/ion_fg2.jpg.)
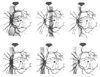 Figure 82-5
The locations of the watershed zones between the medial
and lateral posterior ciliary arteries are shown in gray (shaded
areas) on fluorescein angiograms obtained from patients. (From
Pillanut LE, Harris A, Anderson DR, Greve EL [eds]: Current Concepts on Ocular Blood
Flow in Glaucoma. The Hague, Netherlands, Kugler, 1999.)
Figure 82-5
The locations of the watershed zones between the medial
and lateral posterior ciliary arteries are shown in gray (shaded
areas) on fluorescein angiograms obtained from patients. (From
Pillanut LE, Harris A, Anderson DR, Greve EL [eds]: Current Concepts on Ocular Blood
Flow in Glaucoma. The Hague, Netherlands, Kugler, 1999.)
The orbital veins are devoid of valves. The superior ophthalmic vein (SOV) contains most of the ocular venous outflow. The retina and anterior ON are drained by the central retinal vein (CRV), which empties into the SOV. The choroid is drained by four vortex veins located in each posterior quadrant of the eye; these veins drain into the SOV and the inferior ophthalmic vein (IOV) and have numerous anastomoses between them. Both the SOV and the IOV empty into the cavernous sinus. [10] [16]
The visual cortex is supplied by the posterior cerebral arteries, and the ON radiation is supplied by the middle cerebral arteries in the parietotemporal lobes. Occlusion of both posterior cerebral arteries does not uniformly produce blindness because of collateral circulation through the circle of Willis.[17] Infarction in the visual cortex is instead believed to be embolic or the result of a bilateral "watershed" incident from hypoperfusion in the distal branches of the posterior cerebral circulation.[17] [18] [19] [20]
The anterior portion of the ON is proximal to the lamina cribrosa, an elastic, collagenous tissue through which the ON and the CRA and CRV pass as they enter the optic disk. The anterior portion of the ON includes the superficial nerve fiber layer and the prelaminar region. The prelaminar area is a thick tissue constituting the major part of the optic disk volume.[21] The superficial nerve fiber layer, which is composed of axons extending from the retinal ganglion cells, is anterior to the plane extending across the ON from the peripapillary Bruch membrane. Immediately posterior is the prelaminar region, adjacent to the peripapillary choroid. The laminar region is a transition zone between columns of glial cells and dense connective tissue plates. Astrocytes are predominant in the anterior ON, and oligodendrocytes and microglia are more common in the posterior or retrobulbar ON. Neural fibers transit the laminar region through fenestrations. The retrolaminar region is the posterior portion of the ON and consists of meningeal sheaths and myelinated axons. The ON diameter is enlarged in this area to about 3 mm.
The superificial nerve fiber layer derives its blood supply mainly from arterioles in the retina, although in the temporal regions, it may receive blood from the PCAs. The prelaminar region is perfused by centripetal branches of the peripapillary choroid and vessels from the circle of Zinn-Haller. The circle of Zinn-Haller is not found in every eye.[12] It is controversial whether the region has a choroid-derived source of blood. The laminar region is supplied by centripetal branches from the sPCAs or by the circle of Zinn-Haller, but the sPCAs are the primary input. Longitudinal anastomoses of capillaries can be seen in the prelaminar and laminar regions and may provide some additional protection against ischemic insult, although their functional importance is not clearly known. The retrolaminar, posterior portion of the ON (see Fig. 82-4 ) is perfused by two main vascular supplies. The peripheral centripetal vascular system is the major supply and is found in all ONs. It is formed by recurrent branches of the peripapillary choroid and circle of Zinn-Haller. Pial branches of the CRA and other orbital arteries also contribute. Branches of the pial vasculature run in the septa of the nerve. The axial centrifugal vascular system is formed by small branches from the intraneural part of the CRA and is not present in every eye. [22]
The anterior ON is drained by the CRV. In the superficial nerve fiber layer, small veins converge into the CRV. In the prelaminar and laminar regions, centripetal branches lead into the CRV. The posterior ON is drained by pial veins that drain into the CRV as it exits from the ON.
The capacity of the ON vasculature for autoregulation has potentially important implications in the pathophysiology of ischemic disorders. However, studies of autoregulation of blood flow in the ONH have yielded conflicting results, so studies must be interpreted while bearing in mind the limitations of the measurement techniques. The background behind this controversy has been described in detail by Hayreh.[12] Briefly, interpretation of experimental results depends on the species in which the measurements are made, the location of the measurements, the methods used to measure or estimate blood flow, and the physiologic manipulations used to test autoregulation. Color Doppler methods,[23] [24] applicable in humans, measure velocity, not flow, and cannot distinguish the paraoptic sPCAs from the other sPCAs and the long PCAs.
Blood flow in the ONH is autoregulated within a range of perfusion pressures similar to that in the brain of monkeys and sheep.[27] [28] The mechanism of autoregulation involves nitric oxide.[29] In atherosclerotic monkeys, however, autoregulation was found to be defective.[30] This study did not directly measure blood flow; rather, it measured glucose consumption, and the sample size was small. Nonetheless, in a cat study, ON blood flow was measured directly by autoradiography and found to remain constant in the prelaminar, laminar, and post-laminar ON across a range of systemic mean arterial blood pressure values from 40 to >200 mm Hg.[26]
Blood flow in the ONH measured by laser Doppler flowmetry in 13 healthy volunteers was constant between ocular perfusion pressures of 56 to 80 mm Hg in one study,[31] and preservation of flow at extremely high IOP resulted in a minimal perfusion pressure of 22 mm Hg in another human study.[32] Other investigators found that flow was preserved in the ONH until ocular pressure reached levels of 40 mm Hg. However, 2 of 10 healthy young volunteers in the study failed to demonstrate autoregulation. [33] Using color Doppler imaging, another group showed that flow velocity in the PCAs decreased at extremely high IOP in humans. These findings seem to support speculation that "watershed" areas in the distribution of the PCAs predispose some patients, including otherwise healthy ones with no known vascular disease, to infarction of the anterior portion of the ON when perfusion pressure is decreased, either as a result of decreased systemic blood pressure or because of elevated IOP. At present, however, no clinically available technique can reliably detect such patients.
Both the choroidal and retinal blood vessels supply blood to the retina. The choroid provides oxygen to the outer layers of the retina (where the photoreceptors are located) by diffusion. Blood flow in the choroid is high (≅2000 mL/min/100 g) and oxygen extraction is low (3%) under normal conditions.[34] About 60% to 80% of the retinal oxygen supply comes from the choroid.[10] It was previously thought that the choroid was a "passive" circulation, insignificant for retinal homeostasis. However, its importance in maintenance of a normal retina, particularly in preserving photoreceptor function,[35] has now become clear.[36] The choroid is adrenergically innervated and responds to changes in arterial blood pressure and oxygen or carbon dioxide tension.[37] [38] [39] The autoregulatory mechanism involves nitric oxide and endothelin.[40] [41] [42]
The retinal vessels, which nourish the inner two thirds of the retina, autoregulate in response to changes in arterial PO2 or PCO2 or perfusion pressure similar to that observed in the cerebral vasculature. When compared with the choroid, retinal O2 extraction is high (38%) and blood flow is low (≅40 to 100 mL/min/100 g).[10] Retinal or choroidal blood flow may be altered during anesthesia and surgery. Inhalation of 7% CO2 , which increased arterial PCO2 to 80 mm Hg, increased retinal blood flow by 300% to 400%. Likewise, inhalation of 4% to 6% CO2 increases choroidal blood flow.[10] Inhalation of hypercapnic gas mixtures has been used empirically in humans after retinal arterial occlusion or spasm to increase retinal blood flow.[43]
A decrease in mean arterial blood pressure diminishes ocular perfusion pressure (difference between mean arterial and retinal venous pressure).[10] In nearly all studies, IOP has been considered equivalent to retinal venous pressure. Though not strictly valid,[44] this assumption does not change interpretation of the effects of decreased perfusion pressure on ocular blood flow. Increases in IOP decrease retinal and choroidal blood flow.[37] [38] Under conditions of extremely high IOP in cats (i.e., >40 mm Hg above systolic blood pressure), ocular blood flow virtually ceases. [45] Under such conditions, choroidal blood flow was reduced to 6% and retinal blood flow to 0.6% of baseline. Changes in hematocrit may alter ocular blood flow. Hemodilution (hematocrit changed from 36% ± 1% to 20% ± 1%) increased retinal blood flow by 71% and preserved retinal oxygen delivery while producing a nonstatistically significant 19% decrease in choroidal blood flow.[46] Accordingly, hemodilution has been used in humans to treat CRV occlusion because the increased blood viscosity may contribute to decreased retinal blood flow.[47]
Anesthetics alter ocular blood flow ( Fig. 82-6 ). Both halothane and enflurane produce dose-related increases in retinal and decreases in choroidal blood flow.[34] [48] Decreases in choroidal blood flow parallel decreases in perfusion pressure. The clinical significance of these findings is, however, not yet known.
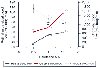 Figure 82-6
Halothane alters retinal and choroidal blood flow in
cats. Note that the changes in retinal blood flow parallel those in the cerebral
cortex. MAC, minimum alveolar concentration. *P
< .0167 versus 0.5 MAC; §P < .0167 versus
1.0 MAC. (From Roth S: The effects of halothane on retinal and choroidal
blood flow in cats. Anesthesiology 76:455–460, 1992.)
Figure 82-6
Halothane alters retinal and choroidal blood flow in
cats. Note that the changes in retinal blood flow parallel those in the cerebral
cortex. MAC, minimum alveolar concentration. *P
< .0167 versus 0.5 MAC; §P < .0167 versus
1.0 MAC. (From Roth S: The effects of halothane on retinal and choroidal
blood flow in cats. Anesthesiology 76:455–460, 1992.)
|
|
|
|
|
|
|
|
|
|
|
|
|