|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Generally speaking, unlike adults, infants and children predominantly experience cardiorespiratory arrest secondarily, rarely as a result of a primary cardiac event.[6] [250] [251] [257] [258] [259] [260] This observation is critical because ACLS interventions directed primarily toward restoration of cardiac function may cause the physician to delay properly directed therapy. For example, a progressive decline in cardiac rate leading to bradycardic arrest is likely to be an ominous sign of hypoxia in infants and children. Treatment with atropine in a futile attempt to increase the heart rate would be tragically misdirected therapy. Furthermore, VF and VT, so common in adults, are uncommon cardiac arrest arrhythmias in infants and small children,[257] [258] although some evidence indicates that VF is not rare in children and adolescents experiencing prehospital cardiac arrest.[261] Thus, application of ACLS is indeed different in these patient populations. Because the cause of cardiac arrest in children is most commonly respiratory and not cardiogenic, the ensuing pathophysiologic events and mode of manifestation of the cardiac arrest state is different, and the subsequent initial efforts at resuscitation must be directed toward respiratory support maneuvers. In addition, of course, equipment, techniques, and drug dosages are of necessity different. All these facts mandate that physicians caring for infants and children have a clear understanding of the role of ACLS interventions in these patients.
The foregoing discussion underscores what is well known to anesthesiologists: definitive control of the airway and oxygenation and ventilation is the most decisive, pivotal, and critical ACLS intervention in pediatric cardiac arrest. As in adults, anesthesiologists ordinarily intubate the trachea for this purpose and then ventilate with such devices as anesthesia bags or bag-valve units with supplemental oxygen. [262] It should be noted that alternative devices such as the esophageal-tracheal Combitube referred to earlier for use in adults is not available in sizes for pediatric application.[262] It should also be noted that with the evolution of experience with LMAs, these devices have been successfully used for neonatal resuscitative efforts in the delivery room setting.[263] [264] [265] However, the LMA should be viewed as an adjunct in this setting, with the airway secured by an endotracheal tube being the gold standard during resuscitation.
ECG monitoring permits immediate recognition of the arrest rhythm or, more likely in infants and children, the "prearrest" rhythm. Prompt intervention and correction in the latter event prevent cardiac arrest that may be irreversible if induced by unrecognized hypoxia and manifested by progressive bradycardia. With this in mind, potentially life-threatening arrhythmias are discussed first, followed by cardiac arrest.
As already discussed, the appearance and progression of slowing of the heart rate in infants and children demand immediate assessment of the cause, first by ruling out the most likely culprit—hypoxia. The bradycardia at this point (before cardiac arrest) is most commonly sinus or junctional in origin. Second- or third-degree heart block may ensue if corrective treatment is not applied. If hypoxia has been ruled out and the bradycardia is accompanied by clinical evidence of impaired perfusion, usually with systemic hypotension, the patient should be treated with epinephrine in a dose of 0.01 mg/kg intravenously, repeated if necessary every 3 to 5 minutes. A 1:10,000 dilution of epinephrine is used for intravenous injections. If the drug is injected into the tracheobronchial tree through an endotracheal tube, 0.1 mg/kg (1:1000 dilution) is recommended. The intraosseous route can also be used if necessary.
Epinephrine is the first choice in treating bradyarrhythmias in infants and children. If the rate does not increase with this drug, atropine can be used in a dose of 0.02 mg/kg and repeated once. An algorithm depicting recommended interventions for pediatric bradyarrhythmias is shown in Figure 78-18 .
In infants and children, supraventricular tachycardia (SVT) may represent ectopic atrial rhythms, atrial flutter or fibrillation, or paroxysmal reentrant tachycardia.[266] Standard pediatric cardiology texts should be referred to for specific diagnostic and therapeutic details. Although SVT is usually well tolerated in most infants and older children, immediate cardioversion may be necessary when heart rates in the range of 240 to 300 beats/min are reached—rates that may result in severe and rapid hemodynamic compromise. In this emergency setting in which rapid SVT causes cardiovascular instability,[267] synchronized cardioversion is the treatment of choice. The initial energy dose is 0.5 J/kg body weight; if necessary, the energy dose is doubled.[254] If conversion to sinus rhythm still does not occur, the diagnosis of SVT should be reconsidered.
In hemodynamically stable children with SVT, adenosine is the drug of choice, as with adults. The drug can be given as a rapid bolus in a dose of 0.1 mg/kg intravenously, ideally injected into the closest port to the hub of the catheter and flushed with 2 to 3 mL of saline. If the
 Figure 78-18
Basic life support (BLS) bradycardia algorithm. ABCs,
airway, breathing, circulation; ALS, advanced life support; CPR, cardiopulmonary
resuscitation. (Redrawn with modification from American Heart Association
in collaboration with International Liaison Committee on Resuscitation: Guidelines
2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care: International
Consensus on Science, Parts 1–12. Circulation 102(Suppl I):I–1, 2000.)
Figure 78-18
Basic life support (BLS) bradycardia algorithm. ABCs,
airway, breathing, circulation; ALS, advanced life support; CPR, cardiopulmonary
resuscitation. (Redrawn with modification from American Heart Association
in collaboration with International Liaison Committee on Resuscitation: Guidelines
2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care: International
Consensus on Science, Parts 1–12. Circulation 102(Suppl I):I–1, 2000.)
Verapamil is not recommended for SVT in an emergency setting because it may induce systemic hypotension and a state of depressed myocardial contractility. [273] [274] [275] Specifically, it is not recommended in this setting for infants younger than 1 year, children with congestive heart failure or myocardial depression, children receiving β-adrenergic-blocking drugs, or children who may have a bypass tract.[254]
In circumstances in which adenosine has been unsuccessful in converting hemodynamically stable SVT, procainamide and amiodarone can be considered ahead of cardioversion.[276] [277] Procainamide, 15 mg/kg, can be delivered over a period of 30 to 60 minutes. Amiodarone, 5 mg/kg delivered over a 20- to 60-minute period, can also be considered. Clinicians are warned not to administer procainamide and amiodarone together.
As with supraventricular bradyarrhythmias, a slow idioventricular rhythm that has not yet produced pulselessness must be considered indicative of severe hypoxia ( Fig. 78-19 ), and treatment must be directed accordingly. Only after this condition has been addressed should rate-accelerating therapy be invoked, as discussed for supraventricular bradycardia.
Despite a common misconception, VT is not always associated with severe hemodynamic compromise. Slow ventricular rates (e.g., 150 to 200 beats/min) may be well tolerated, as defined by the presence of palpable pulses, but they should nonetheless be converted to a normal rhythm. In this setting, synchronized cardioversion is the indicated treatment, beginning at 0.5 J/kg and increasing to progressively higher doses (up to 1 J/kg) if needed. If pharmacologic therapy is undertaken, amiodarone is the drug of choice and is delivered as described for the treatment of SVT. Intravenous procainamide (in doses identical to those for treating SVT) or lidocaine, 1 mg/kg delivered over a period of 4 to 5 minutes, is also an acceptable intervention. Emphasis is placed on proper diagnosis with 12-lead ECGs before treatment if the patient remains hemodynamically stable. However, if the tachycardia is very rapid, as with rates of 300/min or more, and if it is associated with frank cardiovascular collapse (i.e., no palpable pulses), this situation should be treated like VF.
Once pulselessness ensues, therapy must be directed not only at correcting the underlying rhythm disorder but also at maintaining organ viability during the period of cardiorespiratory arrest. Meticulous attention must be paid to ensuring proper CPR technique and the administration of drugs that appear to help support perfusion pressures during cardiac arrest and resuscitation.
In the great majority of pediatric cardiac arrests, bradyasystole is the terminal cardiac electrical activity (see Fig. 78-19 ). Early dominance of the parasympathetic nervous system and α-adrenergic activity may, in part, explain this dominance of bradyasystolic mechanisms as the terminal event in infants.[257] In addition, ventricular tachyarrhythmias (VT/VF) are most commonly seen in infants and children with congenital heart disease.[257] Such children may have cardiac hypertrophy and increased ventricular muscle mass sufficient to provide an anatomic substrate for reentrant wave fronts that initiate and sustain VT or VF.
PEA, as in adults, includes disorders in which some form of cardiac electrical activity is present, but without palpable pulses. In infants and children, this disorder is most commonly idioventricular rhythm at a very slow and irregular rate (bradyasystole) and is the usual predecessor to ventricular asystole. When PEA is present in the form of organized electrical activity (electromechanical dissociation), or even when any electrical activity is still present, an immediate and aggressive effort should be made to identify
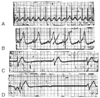 Figure 78-19
A–D, Serial electrocardiograms
in a 5-year-old victim of fatal smoke inhalation. Note the progressive slowing of
the heart rate from sinus tachycardia (A) to a terminal,
very slow idioventricular rhythm (D). (From
Walsh CK, Krongrad E: Terminal cardiac electrical activity in pediatric patients.
Am J Cardiol 51:557, 1983.)
Figure 78-19
A–D, Serial electrocardiograms
in a 5-year-old victim of fatal smoke inhalation. Note the progressive slowing of
the heart rate from sinus tachycardia (A) to a terminal,
very slow idioventricular rhythm (D). (From
Walsh CK, Krongrad E: Terminal cardiac electrical activity in pediatric patients.
Am J Cardiol 51:557, 1983.)
For both PEA and asystole, epinephrine is the drug of choice and is given initially in a dose of 0.01 mg/kg (0.1 mL/kg of a 1:10,000 solution) intravenously or interosseously. Defibrillation is not associated with benefit in PEA or asystole. Emphasis is placed on correcting potentially reversible conditions.
If vascular access has not been achieved, initial treatment with epinephrine can be given through an endotracheal tube. For this purpose, the recommended dose is 0.1 mg/kg of a 1:1000 solution. The dose should be repeated at 3- to 5-minute intervals, as long as arrest persists. Epinephrine is presumed to have the same beneficial actions on elevated perfusion pressure as discussed earlier in the management of adult arrest. In an infant animal model (infant piglets), epinephrine increased cerebral and myocardial blood flow, a finding suggesting that it may have a similar beneficial action in infants and children.[278]
If venous cannulation cannot be achieved quickly, the intraosseous route, preferred over the endotracheal tube route, can be used. This access route is preferred for children 6 years or younger, although it has been successfully used in older children. Figure 78-21 outlines the current algorithm recommended for establishing priorities for vascular access during resuscitation. Alternatives to peripheral venous access should be considered in this setting if reliable access cannot be achieved in three attempts or 90 seconds, whichever comes first.[6] [254]
 Figure 78-20
Asystole and pulseless arrest decision tree. (Redrawn
with modification from Chameides L [ed]: Textbook of Pediatric Advanced Life Support.
Dallas, American Heart Association, 2000.)
Figure 78-20
Asystole and pulseless arrest decision tree. (Redrawn
with modification from Chameides L [ed]: Textbook of Pediatric Advanced Life Support.
Dallas, American Heart Association, 2000.)
Two main types of devices are currently available for intraosseous cannulation: the intraosseous infusion needle and the Jamshidi-type bone marrow aspiration needle. If either of these needles is not available in the emergency setting, an 18-gauge spinal needle with a stylet may be considered for this use. Several publications describe the history, technique, and application of intraosseous infusion in the resuscitation of infants and children.[279] [280] [281] [282] Whichever needle is used, it is inserted perpendicular to the skin into the tibia approximately one fingerbreadth below the tubercle on the anteromedial surface. The needle is advanced with a boring-type motion until the marrow cavity is entered, as evidenced by a distinct loss of resistance as the needle passes through the bony cortex.[282] The stylet is removed, and bone marrow is aspirated into a saline-filled syringe. The needle is then flushed with saline and connected to an intravenous administration set. Any fluid or drug needed for resuscitation can be administered by this route. Although fluid should flow freely through the needle, administration of fluid with an intravenous pump can be considered if care is taken to ensure absence of subcutaneous infiltration.
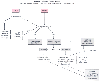 Figure 78-21
Priorities for vascular access. CPR, cardiopulmonary
resuscitation; ET, endotracheal. (Redrawn with modification from Chameides
L [ed]: Textbook of Pediatric Advanced Life Support. Dallas, American Heart Association,
2000.)
Figure 78-21
Priorities for vascular access. CPR, cardiopulmonary
resuscitation; ET, endotracheal. (Redrawn with modification from Chameides
L [ed]: Textbook of Pediatric Advanced Life Support. Dallas, American Heart Association,
2000.)
In CPR of infants and children, it is recommended that a rapid glucose test be performed and glucose be injected if hypoglycemia is present.[6] [254] For infants and children who do not respond to resuscitative therapy, glucose administration should also be considered. However, as expressed earlier in the discussion of adult cardiac arrest, routine use of glucose-containing solutions should be avoided because of a potentially adverse impact on neurologic outcome and because glucose administration may result in hyperglycemia and secondary osmotic diuresis. If glucose is necessary, the dose is 0.5 to 1.0 g/kg intravenously in a solution of 25% dextrose in water (2 to 4 mL/kg) for infants and children, preferably given as a continuous infusion rather than a bolus to avoid a sudden increase in serum osmolality.[6] [254] The commercially available 50% dextrose in water solution can be diluted 1:1 with sterile water for this purpose. For neonates, in general, the concentration of glucose administered should not exceed 12.5%, thus necessitating further dilution of the 50% dextrose solution.
The same concerns discussed earlier regarding sodium bicarbonate therapy in adults also apply in pediatric patients. If it is used, 1 mEq/kg can be given initially and 0.5 mEq/kg at 10-minute intervals thereafter, unless arterial blood measurements of pH, PaCO2 , and base deficit are available and indicate a different dosage scheme.[254] Likewise, no available evidence supports the continued use of calcium salts for cardiac arrest in infants and children. The use of calcium chloride should be reserved for the acute treatment of hyperkalemia, hypermagnesemia, hypocalcemia, or calcium channel blocking drug toxicity. In these clinical situations, 0.2 to 0.25 mL/kg of 10% calcium chloride (5 to 7 mg/kg elemental calcium) can be given and repeated once in 10 minutes.[254]
As indicated earlier, this form of cardiac arrest is relatively uncommon in infants and children and is most likely to be seen when congenital heart disease and increased cardiac muscle mass are present to provide the anatomic electrophysiologic milieu for maintaining reentrant wave fronts. Defibrillation should be performed as soon as possible, and all defibrillators should be equipped with electrode paddles suitable for pediatric age groups. Pediatric defibrillator
| Drug | Dose | Remarks |
|---|---|---|
| Adenosine | Up to 0.1 mg/kg | Rapid IV bolus |
|
|
Maximum single dose: 12 mg |
|
| Atropine sulfate | 0.02 mg/kg per dose | Minimum dose: 0.1 mg |
|
|
|
Maximum single dose: 0.5 mg in child, 1.0 mg in adolescent |
| Calcium chloride (10%) | 20 mg/kg per dose | Give slowly |
| Dopamine hydrochloride | 2–20 µg/kg/min | α-Adrenergic action dominates at 15–20 µg/kg/min |
| Epinephrine | IV/IO: 0.01 mg/kg (1:10,000) | Be aware of effective dose of preservatives administered (if preservatives are present in epinephrine preparation) when high doses are used |
| For bradycardia | ET: 0.1 mg/kg (1:1000) |
|
| For asystolic or pulseless arrest | First dose: | Be aware of effective doses of preservative administered (if preservatives present in epinephrine preparation) when high doses are used |
|
|
IV/IO: 0.01 mg/kg (1:10,000) |
|
|
|
ET: 0.1 mg/kg (1:1000) |
|
|
|
Doses as high as 0.2 mg/kg may be effective |
|
|
|
Subsequent doses: |
|
|
|
IV/IO/ET: 0.1 mg/kg (1:1000) |
|
|
|
Doses as high as 0.2 mg/kg may be effective |
|
| Epinephrine infusion | Initially at 0.1 µg/kg/min | Titrate to desired effect (0.1–1.0 µg/kg/min) |
|
|
Higher infusion dose used if asystole present |
|
| Lidocaine | 1 mg/kg per dose |
|
| Lidocaine infusion | 20–50 µg/kg/min |
|
| Sodium bicarbonate | 1 mEq/kg per dose or 0.3 × kg × base deficit | Infuse slowly and only if ventilation is adequate |
| ET, endotracheal route; IO, intraosseous route; IV, intravenous route. | ||
| (From American Heart Association in collaboration with International Liaison Committee on Resuscitation: Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care: International Consensus on Science, Parts 1–12. Circulation 102(Suppl I):I–1, 2000.) | ||
As long as pulseless VT or VF persists, epinephrine should be given in doses described for PEA-related cardiac arrest in an attempt to increase cerebral and myocardial perfusion pressure during CPR. A decision tree for intervention in cardiorespiratory arrest in infants and children is presented in Figure 78-20 .
During circumstances in which persistent or recurrent VT or VF is present, amiodarone (5 mg/kg) or lidocaine (1 mg/kg) may be administered. Thirty to sixty seconds of CPR should follow the administration of any antiarrhythmic drug and then defibrillation attempted again.
One of the most difficult aspects of pediatric resuscitation is determination of correct drug dosages in the midst of the crisis, especially if one deals with cardiac arrest in infants and children infrequently. Various schemes have been proposed for simplifying this need. Table 78-4 incorporates a convenient scheme for selecting the proper dose of drug to be injected based on body weight. It includes the major resuscitative drugs (i.e., epinephrine, atropine, and lidocaine), along with additional drugs that could be used in some cardiac arrests or other pediatric emergencies. The availability of a resuscitation team knowledgeable in pediatric resuscitation is critical for the proper management and resuscitation of a child victim of cardiac and near-cardiac arrest.
|
|
|
|
|
|
|
|
|
|
|
|
|