|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For severely ill patients, indirect calorimetry seems the most feasible method. The measurement of four independent quantities is required for this procedure[130] : daily intake of fat, carbohydrate, and protein; daily nitrogen excretion; daily oxygen consumption (DVO2 ); and daily CO2 production (DVCO2 ). For bedridden patients who are completely at rest during the entire 24 hours, three to five measurements of REE (40 to 60 minutes' duration) may be extrapolated to obtain a reasonably good estimate of total energy expenditure (TEE) for the 24-hour period. Van Lanschot and associates[131] compared the Harris-Benedict equation with the accurate measurement of TEE by continuous indirect calorimetry. Despite the overestimation of TEE when caloric needs were calculated by formula, they remarked that the difficulties associated with continuous indirect calorimetry left "unresolved, whether or not indirect calorimetry is actually to be preferred in daily clinical practice."[131]
The usual approach is to measure DVO2 , DVCO2 , and nitrogen excretion. Energy requirements must be adjusted for variations resulting from difference in body size. The basal metabolic rate refers to energy expenditure at a specific time in the morning, soon after awakening and approximately 14 hours after the last meal, measured at thermal neutrality. This discussion refers to basal energy expenditure (BEE) instead of basal metabolic rate.
Heat production per square meter of body surface area per unit time decreases steadily as humans approach puberty; then energy expenditure more gradually decreases as age increases. This decrease is approximately 1% to 2% per decade in adults (20 to 75 years old).[132]
The Harris-Benedict equation is an empirically derived parametric
equation with variables that reflect the relative contributions to overall heat production
per square meter body surface area of activity, age, sex, and body size:
For males:
BEE = 66 + (13.7 × wt in kg) + (5 ×
ht in cm) − (6.8 × age)
For females:
BEE = 655 + (9.6 × wt in kg) + (1.7 ×
ht in cm) − (4.7 × age)
BEE is the basal energy expenditure in kilocalories per day.
To apply this equation to nutritional requirements, the BEE is multiplied by activity and injury factors to arrive at a daily nutrition requirement in kilocalories per day, or TEE. Activity factors vary from 1 for a patient on bed rest to 1.3 for an ambulatory patient. Injury factors vary from 1 to 1.2 for minor surgery to 1.8 for major sepsis ( Table 77-5 ). For each degree above 37.2°C, the daily nutritional requirement is multiplied by 1.07. An important reason for excessive energy administration is using TEE based on weight and on estimates of BEEs rather than on measured metabolic expenditures.[131] [133] Lean tissue repletion is maximal at 4 to 6 g/day of nitrogen in the malnourished, nonstressed patient receiving energy at 1.75 × BEE (estimated BEE) and protein at 1.5 g/kg/day.[134] Any positive energy balance beyond this is used in fat synthesis. In the past, nutritional support was used with very high multiples of BEE to attempt to force positive nitrogen balance. Current practice is to prescribe energy (e.g., glucose, lipids) to meet measured energy requirements and separately prescribe proteins (i.e., amino acids) to meet measured nitrogen excretion. [135] For obese patients, hypocaloric feeding is frequently prescribed to induce consumption of endogenous lipids for energy requirements.
|
|
Resting Energy Expenditure | |
|---|---|---|
| Condition | Calories (kcal/24 hr) * | Percent of Normal * |
| Normal man | 1800 | 100 |
| Simple starvation (20 days) | 1080 | 60 |
| Postoperative | 1800 | 100 |
| Multiple fractures | 2160 | 120 |
| Major sepsis | 2520 | 140 |
| 60% Burn |
|
|
| In 21°C environment | 3600 | 200 |
| In 25°C environment | 3819 | 212 |
| In 33°C environment | 3342 | 185 |
| From Popp MB, Brennan MF: Metabolic response to trauma and infection. In Fischer JE (ed): Surgical Nutrition. Boston, Little, Brown, 1983, p 492. | ||
Energy expenditure is more accurately assessed by measuring DVO2
and DVCO2
. When urinary nitrogen (UN)
is measured, the formula described by Weir[136]
can be used to calculate the amount of nonprotein energy expended in kilocalories:
Energy expenditure/unit of time = 3.94 DVO2
+ 1.11 DVCO2
− 2.17 UN
In the equation, DVO2
and DVCO2
are in liters per 24 hours at standard temperature and pressure and dry (STPD) conditions,
and UN is in grams per 24 hours.
The technique of indirect calorimetry has been used to measure metabolic rate. It has long been recognized that these measurements also can give information on the type and the rate of fuel oxidation within the body. This latter information is based on a number of assumptions, which may not hold in the presence of two metabolic processes: gluconeogenesis and lipogenesis.[137]
Resting energy expenditure includes BEE and the thermal effect
resulting from food intake as well as nonshivering thermogenesis in response to environmental
temperature alterations. Infusing amino acids increases energy expenditure by 10%
over 24 hours in normal subjects and in postoperative patients, whereas glucose and
lipid infusions have little effect.[138]
[139]
Increased energy expenditure, however, occurs when carbohydrates are given in excess
of energy requirements. REE can be initially estimated from the following formula
[140]
after indirect calorimetry measurements of
oxygen consumption and carbon dioxide production:
REE = 3.94 × DVO2
+ 1.11 × DVCO2
Resting energy expenditure can then be adjusted for protein metabolism by measuring
the UN in grams per day.
The adjusted metabolic expenditure equals REE − 2.17 × UN. A 2-hour UN measurement can be substituted for a 24-hour collection.[141] UN excretion is calculated from measured urinary urea measurements as outlined in Table 77-6 .
The REEs of a hospitalized patient are usually a balance between the decrease that normally occurs in association with any weight loss and nutritional depletion from partial starvation and any increase that results from disease or injury. An average increase of 13% in energy expenditure is associated with each degree celsius of fever.
The RQ is the ratio of DVCO2 divided by DVO2 (DVCO2 /DVO2 ). Normally, the RQ ranges between 0.7 (lipid oxidation) and 1.0 (glucose oxidation) and varies with substrate oxidation. The RQ moves toward 1 with dietary carbohydrate loading and decreases to 0.7 with prolonged fasting, demonstrating almost complete fat oxidation during the starved state. With lipogenesis from excess caloric intake, RQ becomes greater than 1.0. The RQ measurement also reflects gas exchange from protein metabolism. UN excretion is therefore determined, and the contribution of protein combustion to gas exchange is calculated. This correction yields the "nonprotein RQ."
| Urine urea nitrogen = 80% of total urine nitrogen across a wide range |
| 1 g (16.6 mmol) urea = 28/60 g of nitrogen (mol wt of urea = 60) |
| Total body water = 60% body wt in kg |
| Urea equally distributed throughout body water: |
| 1. 24-hr urine urea in g × 28/60 × 6/5 * = X × 0.56 = (A) g |
| 2. Measure of proteinuria, if any = Y × 4/25 † = Y × 0.16 = (B) g |
| 3. Correction for any rise of blood urea assuming no change in body weight in kg |
| Rise in blood urea (in 24 hr) = Z g · 1-1 |
| Z g × 60% body wt × 28/60 = Z × body wt × 0.28 = (C)g |
| (A) ++ (B) ++ (C) = nitrogen loss = minimal nitrogen requirement |
| From Lee HA: Fluid balance and parenteral feeding. In Nunn JF, Utting JE, Brown BR (eds): General Anaesthesia. London, Butterworth, 1989, p 1213. |
Measurement of DVO2
,
DVCO2
, and UN excretion permits determination
of individual fuel sources oxidized and calculation of the total quantity of heat
produced.[142]
The RQ for metabolism of carbohydrates
equals 1:
C6
H12
O6
+ 6 O2
= 6 H2
O + 6 CO2
RQ = 6 DVCO2
/6
DVO2
= 1.0
In oxidation of fat, the RQ is less than 1:
Palmitate + 23 O2
= 16 H2
O
+ 16 CO2
RQ = 16 DVCO2
/23
DVO2
= 0.7
Cells can oxidize multiple substrates concurrently and can convert glucose into fat and glycogen. An RQ derived from gas analysis cannot provide precise information concerning the metabolic pool. However, the RQ estimates the relative contributions of glucose, lipids, and proteins, as well as lipogenesis from overfeeding.
The most common methods for measurement of the respiratory quotient use a closed-circuit or open-circuit technique. The closed-circuit method uses a spirometer, filled with oxygen with a CO2 absorber placed in the circuit. The rate of disappearance of oxygen from the spirometer equals the oxygen consumption, and mean expired CO2 concentration times the minute volume equals the CO2 production. The open-circuit method uses a set of one-way valves to direct expired air into a collecting container (classically, a Douglas bag or a Tissot spirometer). During a carefully timed collecting period, the volume and the composition of the expired gas are measured, and the rates of DVO2 and DVCO2 are determined by the difference between the concentrations of inspired oxygen and carbon dioxide and the expired oxygen and carbon dioxide collected. A correction (Haldane) is used to account for the difference between inspired and expired volumes. Because the Haldane correction depends on a conservation of nitrogen mass between inspired and expired gas, it becomes inaccurate at low levels of inspired nitrogen (i.e., high fraction of inspired oxygen [FIO2 ] levels).
Kinney and colleagues[143] designed a noninvasive system for nonintubated patients that consisted of a clear plastic head canopy (with no attachment to the face or airway) ventilated with air that is continuously analyzed for measurement of DVO2 and DVCO2 . REE of hospitalized patients can be determined by obtaining average gas-exchange measurements from five 30- to 45-minute runs during each waking day ( Fig. 77-20 ).
Commercial instruments with attached CO2 and oxygen analyzers that have been developed to measure DVO2 and DVCO2 in patients can be used in the operating room and intensive care unit. Several problems are associated with validation of these instruments and in performing gas exchange measurements during mechanical ventilation.[144] [145] The closed-circuit devices function well on mechanically ventilated patients because they are not affected by FIO2 . The open circuit devices can be used for patients breathing spontaneously through their native
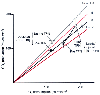 Figure 77-20
Alterations in gas exchange caused by total parenteral
nutrition (TPN) are shown (mean ± SD). Glucose was the primary source of
nonprotein calories, and two responses are seen. Depleted patients show a respiratory
quotient (RQ) above 1.0 with a small increase in V̇O2
,
and hypermetabolic patients have an RQ less than 1.0 with a marked increase in V̇O2
.
Both groups show a large increase in V̇CO2
.
(Adapted from Askanazi J, Carpentier YA, Elwyn DH, et al: Influence of
total parenteral nutrition on fuel utilization in injury and sepsis. Ann Surg 191:40,
1980.)
Figure 77-20
Alterations in gas exchange caused by total parenteral
nutrition (TPN) are shown (mean ± SD). Glucose was the primary source of
nonprotein calories, and two responses are seen. Depleted patients show a respiratory
quotient (RQ) above 1.0 with a small increase in V̇O2
,
and hypermetabolic patients have an RQ less than 1.0 with a marked increase in V̇O2
.
Both groups show a large increase in V̇CO2
.
(Adapted from Askanazi J, Carpentier YA, Elwyn DH, et al: Influence of
total parenteral nutrition on fuel utilization in injury and sepsis. Ann Surg 191:40,
1980.)
|
|
|
|
|
|
|
|
|
|
|
|
|