|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A lack of food has profound effects on the normal flow of nutrients in the body. The immediate result of food deprivation is decreased intake of glucose. Glucose is vital to the animal for survival because certain cells, such as erythrocytes, cells of the renal medulla, and cells of the central nervous system, have an absolute requirement for glucose, amounting to approximately 180 g/day ( Fig. 77-2 ). Despite reduced sugar intake, the circulating blood glucose concentration varies little because humans adapt to decreased glucose intake during starvation by two mechanisms that maintain the plasma glucose concentration. First, fatty acids mobilized from triglyceride stores in adipose tissue are used as an alternative fuel to glucose in tissues that can oxidize fats. This use of alternative fuel for energy production lowers the requirements for glucose, thereby decreasing the demand for more glucose at a time when input through feeding is not available. Second, several adaptations in intracellular glucose metabolism occur that result in an inhibition of glucose-using pathways and a stimulation of glucose-producing pathways ( Fig. 77-3 ). Initially, to maintain the plasma glucose concentration, glycogen is broken down. The loss of glycogen is rapid and significant. Within 48 hours of starvation, rats show a 99.5% loss of liver glycogen and a 70.3% loss of carcass glycogen. However, the amount of glucose released by glycogenolysis is insufficient to sustain the energy needs of the whole body for more than a short period. The liver and, to a certain extent, the kidneys have the ability to synthesize glucose from different carbon sources by means of the process of gluconeogenesis ( Fig. 77-4 ). Glucose is synthesized primarily from glycerol, lactate, pyruvate, and certain amino acids, particularly alanine. Lactate and pyruvate are released by peripheral tissues, particularly skeletal muscle. Lactate provides 60% to 70% of the glucose carbon used for gluconeogenesis.
 Figure 77-2
General scheme of fuel metabolism in normal, fasted humans,
emphasizing the central position of the liver as a metabolic transformer. Two primary
fuel sources are shown: muscle and adipose tissue. Three types of fuel consumers
are shown: nerve, including brain; pure glycolyzers producing lactate (e.g., red
blood cells, white blood cells); and the remainder of the body (e.g., heart, kidney,
muscle), which can use fatty acids and ketones. The brain can also use ketone bodies
after injury and in response to starvation. (Adapted from Cahill GJ Jr:
Starvation in man. N Engl J Med 282:668, 1970.)
Figure 77-2
General scheme of fuel metabolism in normal, fasted humans,
emphasizing the central position of the liver as a metabolic transformer. Two primary
fuel sources are shown: muscle and adipose tissue. Three types of fuel consumers
are shown: nerve, including brain; pure glycolyzers producing lactate (e.g., red
blood cells, white blood cells); and the remainder of the body (e.g., heart, kidney,
muscle), which can use fatty acids and ketones. The brain can also use ketone bodies
after injury and in response to starvation. (Adapted from Cahill GJ Jr:
Starvation in man. N Engl J Med 282:668, 1970.)
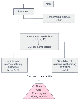 Figure 77-3
Adaptations in glucose metabolism during starvation.
AT, adipose tissue; CNS, central nervous system; L, liver; K, kidney; M, muscle.
Figure 77-3
Adaptations in glucose metabolism during starvation.
AT, adipose tissue; CNS, central nervous system; L, liver; K, kidney; M, muscle.
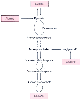 Figure 77-4
Gluconeogenesis from various precursors. The diagram
shows the stages in which amino acids (e.g., alanine), glycerol, and lactate join
the pathway of gluconeogenesis. The reactions common to gluconeogenesis and glycolysis
are those indicated by the straight arrows; the downward
arrows show the direction of gluconeogenesis, the upward
arrows the direction of glycolysis. The curved arrows
represent the reactions (i.e., pyruvate carboxylase, phosphoenolpyruvate carboxykinase,
fructose-1,6-diphosphatase, and glucose-6-phosphatase) circumventing the energy barriers
obstructing the direct reversal of glycolysis. (Adapted from Biebuyck JF:
Anaesthesia and hepatic metabolism: Current concepts of carbohydrate homeostasis.
Anesthesiology 39:188, 1973.)
Figure 77-4
Gluconeogenesis from various precursors. The diagram
shows the stages in which amino acids (e.g., alanine), glycerol, and lactate join
the pathway of gluconeogenesis. The reactions common to gluconeogenesis and glycolysis
are those indicated by the straight arrows; the downward
arrows show the direction of gluconeogenesis, the upward
arrows the direction of glycolysis. The curved arrows
represent the reactions (i.e., pyruvate carboxylase, phosphoenolpyruvate carboxykinase,
fructose-1,6-diphosphatase, and glucose-6-phosphatase) circumventing the energy barriers
obstructing the direct reversal of glycolysis. (Adapted from Biebuyck JF:
Anaesthesia and hepatic metabolism: Current concepts of carbohydrate homeostasis.
Anesthesiology 39:188, 1973.)
More than 35 years ago, an interorgan cycle (i.e., Cori cycle) accounting for the flow of glucose carbon during starvation was proposed. Glucose taken up by peripheral organs is converted to lactate, which enters the blood and is returned to liver. The lactate is taken up by the liver and is synthesized to glucose ( Fig. 77-5 ).
The amount of lactate that can be produced and cleansed by the liver may be extremely high. The normal liver can clear up to 400 g of lactate per day. Lactate production by skeletal muscle must increase to provide the necessary gluconeogenic precursors for the maintenance of sustained rates of gluconeogenesis in starvation or diabetes. Lactate arises from the reduction of tissue pyruvate.
Lactate production occurs whenever the rate of pyruvate production from glycolysis exceeds glucose oxidation by the mitochondria. For increased lactate production to be important physiologically, mitochondrial glucose oxidation must decrease under conditions known to result in increased gluconeogenesis. In humans, whole-body glucose oxidation is inhibited 90% in starvation, 70% in type 1 diabetes mellitus, and 40% in non-insulin-dependent (type 2) diabetes mellitus. Each of these conditions is associated with enhanced rates of gluconeogenesis (see Chapter 25 ).
At the enzymatic level, the pyruvate dehydrogenase (PDH) complex catalyzes the first irreversible reaction in the
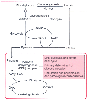 Figure 77-5
Role of inhibition of skeletal muscle glucose oxidation
in starvation and diabetes. CoA, coenzyme A; NAD, oxidized form of nicotinamide
adenine dinucleotide; NADH, reduced form of nicotinamide adenine dinucleotide; OAA,
oxaloacetic acid.
Figure 77-5
Role of inhibition of skeletal muscle glucose oxidation
in starvation and diabetes. CoA, coenzyme A; NAD, oxidized form of nicotinamide
adenine dinucleotide; NADH, reduced form of nicotinamide adenine dinucleotide; OAA,
oxaloacetic acid.
Release of amino acids from skeletal muscle is intimately related to glucose homeostasis because amino acids represent an important precursor for gluconeogenesis. Most amino acids are released from muscle in proportion to their concentration in muscle proteins.[7] However, the exceptions are alanine and glutamine, which are released in excess of their concentration in muscle proteins. These observations implied that de novo synthesis of alanine and glutamine occurred in skeletal muscle. Because alanine is used by the liver and kidney as a major substrate for gluconeogenesis, Felig and colleagues[8] proposed a glucose-alanine cycle as a means of transferring amino nitrogen
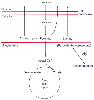 Figure 77-6
Site of action of dichloroacetate (DCA) in mammalian
cells. DCA activates pyruvate dehydrogenase, thereby increasing the flux of C3 compounds
into the tricarboxylic acid cycle and decreasing the release of lactate, pyruvate,
and alanine into the circulation. (Adapted from Blackshear PJ, Fang LST,
Axelrod L: Treatment of severe lactic acidosis with dichloroacetate. Diabetes Care
5:391, 1982.)
Figure 77-6
Site of action of dichloroacetate (DCA) in mammalian
cells. DCA activates pyruvate dehydrogenase, thereby increasing the flux of C3 compounds
into the tricarboxylic acid cycle and decreasing the release of lactate, pyruvate,
and alanine into the circulation. (Adapted from Blackshear PJ, Fang LST,
Axelrod L: Treatment of severe lactic acidosis with dichloroacetate. Diabetes Care
5:391, 1982.)
 Figure 77-7
Mechanism regulating pyruvate dehydrogenase (PDH) complex
activity in starvation and diabetes. FA, fatty acid; KAP, kinase activator protein.
Figure 77-7
Mechanism regulating pyruvate dehydrogenase (PDH) complex
activity in starvation and diabetes. FA, fatty acid; KAP, kinase activator protein.
Although the proposed glucose-alanine cycle allows for the transfer
of nitrogen groups derived from amino acid catabolism to liver, it does not account
for a net flow of carbon from protein to carbohydrate. Alanine and glutamine are
also synthesized from other amino acids. In the postabsorptive state, about 40%
of the circulating plasma alanine is derived from endogenous proteins, whereas 60%
is derived from de novo synthesis in humans.[9]
At least 20% of the nitrogen required for the de novo alanine
|
|
Overnight Fast | Four-Day Fast | ||
|---|---|---|---|---|
| Substrate | Concentration (µmol/mL) | Available Energy (%) | Concentration (µmol/mL) | Available Energy (%) |
| Nonesterified fatty acids | 0.42 | 9 | 1.15 | 20 |
| Triacylglycerol | 1.0 | 65 | 1.0 | 54 |
| Glucose | 4.7 | 25 | 3.6 | 16 |
| Lactate | 0.5 | 1 | 0.5 | 1 |
| Ketone bodies | 0.03 | 1 | 2.9 | 9 |
| From Hawkins RA, Vina JR: Lipid and ketone body metabolism in man. Clin Anaesthesiol 1:559, 1983. | ||||
If requirements for glucose carbon continued unabated, protein breakdown would result in a severe depletion of vital proteins. However, ketone body concentrations rise in long-term starvation or diabetes mellitus. Ketone bodies are derived from the incomplete oxidation of fatty acids in the liver. The brain adapts to using alternative fuels such as ketone bodies as a primary energy source, rather than glucose. By using alternative fuels, the demand for glucose is further reduced ( Table 77-2 ). An important consequence of this adaptation is that the breakdown of muscle is no longer required to maintain the flow of alanine to the liver for gluconeogenesis ( Fig. 77-8 ). The net result is that muscle proteins are spared further degradation. The adaptive ability of the organism to use alternative fuels instead of glucose is of fundamental importance to the survival of the animal, because otherwise, protein function would eventually be compromised.
These adaptive processes are under hormonal control, which regulates the flow of glucose carbon to ensure adequate levels of glucose in the blood. These hormones can be broadly categorized into two groups: anabolic hormones and catabolic hormones. At any given time, the net effect of the hormones, whether to break down or to store fuels, depends on the ratio of concentrations of each of the hormones to each other, as well as on their absolute concentration. The principal anabolic hormone is insulin. Insulin is of major importance in fuel storage, promoting the deposition of glycogen, triglycerides, and proteins. At basal levels, insulin has an important anticatabolic role in restraining glycogenolysis, gluconeogenesis, and lipolysis. Growth hormone (GH) is also anabolic, but only with respect to protein metabolism, in which GH stimulates amino acid transport and protein synthesis. Some studies have suggested that parts of the anabolic effects of GH are mediated by a faster-term insulin-like growth factor-1.[11] The major catabolic hormones are glucagon, cortisol, and catecholamines. Individually, none of these hormones totally opposes the
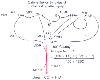 Figure 77-8
Use of metabolic fuels during fasting. The energy fuel
substrate flux shown in terms of calories per day has been calculated from experimental
data. Protein loss is equal to approximately 16.6 g of nitrogen. Aa, amino acid;
ADP, adenosine diphosphate; ATP adenosine triphosphate; FFA, free fatty acid; Glc,
glucose; KB, ketone body; RQ, respiratory quotient; Tg, triglyceride. (Adapted
from Blackburn GL, Phinney SD: Lipid metabolism in injury. In
Burke JF [ed]: Surgical Physiology. Philadelphia, WB Saunders, 1983, p 113.)
Figure 77-8
Use of metabolic fuels during fasting. The energy fuel
substrate flux shown in terms of calories per day has been calculated from experimental
data. Protein loss is equal to approximately 16.6 g of nitrogen. Aa, amino acid;
ADP, adenosine diphosphate; ATP adenosine triphosphate; FFA, free fatty acid; Glc,
glucose; KB, ketone body; RQ, respiratory quotient; Tg, triglyceride. (Adapted
from Blackburn GL, Phinney SD: Lipid metabolism in injury. In
Burke JF [ed]: Surgical Physiology. Philadelphia, WB Saunders, 1983, p 113.)
|
|
|
|
|
|
|
|
|
|
|
|
|