|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Although a complete discussion of congenital heart disease is not possible here, various abnormalities that produce significant alterations in oxygenation, perfusion, and myocardial function after birth are worthy of attention ( Table 76-1 ; also see Chapter 51 ). The more common lesions include obstruction to systemic outflow (i.e., congenital aortic stenosis and coarctation of the aorta) and lesions that alter the adequacy of pulmonary blood flow (i.e., tricuspid or pulmonary atresia or tetralogy of Fallot). Many of these lesions are associated with other abnormalities that facilitate mixing or diversion of blood flow from one circuit to the other to permit even marginal survival. Patent fetal conduits such as the ductus arteriosus or foramen ovale may exist in isolation or in association with other "shunt" lesions, and in some circumstances, rather than helping survival, they may become a source of cardiac decompensation. [7]
Newborns with significant congenital heart disease commonly present with either cyanosis or congestive heart failure (CHF). It is important to recognize that the degree of dysfunction changes during the first 2 to 3 months of life because PVR decreases to adult levels.[30] As this resistance falls, left-to-right shunts can increase, and the symptoms of CHF become more apparent. In the newborn, the usual signs and symptoms of CHF include poor feeding, irritability, sweating, tachycardia, tachypnea, decreased peripheral pulses, poor cutaneous perfusion, and hepatomegaly. Cyanosis may indicate structural cardiac disease; however, respiratory disease, increased PVR (persistent pulmonary hypertension), and methemoglobinemia must also be considered ( Table 76-2 ). [7]
The diagnosis of congenital heart disease can be made on the basis
of the physical examination, electrocardiogram (ECG), chest radiograph, and echocardiogram.
Cardiac catheterization may be performed during initial stabilization as an interventional
therapy or as a diagnostic tool before either definitive or palliative cardiac surgery.
Magnetic
| Age | Heart Rate (Beats/min) | Systolic Blood Pressure (mm Hg) | Diastolic Blood Pressure (mm Hg) | Stroke Volume (mL/Beat) | Cardiac Index (L/min/m2 ) | Oxygen Consumption (mL/kg/min) | Hemoglobin Concentration (g/dL) | P50 (mm Hg) |
|---|---|---|---|---|---|---|---|---|
| Term newborn | 133 ± 18 | 80 ± 16 | 46 ± 16 | 4.5 ± 5.0 | 2.5 ± 0.6 | 6.0 ± 1.0 | 16.5 ± 1.5 | 18 |
| 6 mo | 120 ± 20 | 89 ± 29 | 60 ± 10 | 7.4 ± 2.0 | 2.0 ± 0.5 | 5.0 ± 0.9 | 11.5 ± 1.0 | 24 |
| 12 mo | 120 ± 20 | 96 ± 30 | 66 ± 25 | 11.5 ± 3.0 | 2.5 ± 0.6 | 5.2 ± 0.9 | 12.0 ± 0.75 | - |
| 2 yr | 105 ± 25 | 99 ± 25 | 64 ± 25 | 16.9 ± 4.5 | 3.1 ± 0.7 | 6.4 ± 1.2 | 12.5 ± 0.5 | 27 |
| 5 yr | 90 ± 10 | 94 ± 14 | 55 ± 9 | 27.8 ± 7.5 | 3.7 ± 0.9 | 6.0 ± 1.1 | 12.5 ± 0.5 | - |
| 12 yr | 70 ± 17 | 113 ± 18 | 59 ± 10 | 53.5 ± 14.5 | 4.3 ± 1.1 | 3.3 ± 0.6 | 13.5 ± 1.0 | - |
| Adult | 75 ± 5 | — | — | 85.5 ± 6.0 | 3.7 ± 0.3 | 3.4 ± 0.6 | 14.0 ± 1.0 | 27 |
| P50 , oxygen half-saturation pressure of hemoglobin. | ||||||||
| From Crone RK: Pediatric Intensive Care. ASA Refresher Courses in Anesthesiology, vol. 9. Philadelphia, JB Lippincott, 1981. | ||||||||
| Cyanotic congenital heart lesions |
| Tetralogy of Fallot |
| Transposition of the great arteries |
| Hypoplastic left heart syndrome |
| Pulmonary atresia with intact ventricular septum |
| Single ventricle |
| Total anomalous pulmonary venous return |
| Tricuspid atresia |
| Congenital heart lesions manifested as congestive heart failure |
| Ventricular septal defect |
| Patent ductus arteriosus |
| Hypoplastic left heart syndrome |
| Critical aortic stenosis |
| Coarctation of the aorta |
The initial medical treatment of congenital heart disease is aimed at relieving CHF, improving systemic perfusion, and maintaining pulmonary blood flow. In some situations (i.e., hypoplastic left heart syndrome, aortic stenosis, or atresia), patency of the ductus arteriosus may be crucial to provide perfusion to the body. In these cases, PGE1 infusion has proved useful in maintaining patency of the ductus arteriosus until definitive surgical correction can be performed (see the discussion of pharmacology later in this chapter).[31] [32]
Acute circulatory failure is defined as any clinical condition in which systemic blood flow is inadequate to meet the metabolic demands of the body. [33] The clinical syndrome of shock includes the signs and symptoms of both an inadequate circulation and an attempt to compensate
In children, the most common cause of circulatory failure is hypovolemia from excessive volume loss. This condition produces decreased ventricular preload, which reduces stroke volume and cardiac output. Causes of acute hypovolemia are (1) blood loss (trauma, gastrointestinal [GI] bleeding), (2) plasma loss (capillary leak syndrome associated with sepsis, hypoproteinemia, burns, peritonitis), and (3) water loss (most commonly vomiting and diarrhea, glycosuric diuresis in diabetic ketoacidosis [DKA]). Children with acute hypovolemia usually have extreme peripheral vasoconstriction with cool, poorly perfused extremities. Peripheral pulses may be thready, and the capillary refill time may be prolonged.
Intravascular hypovolemia can also result from decreased PVR associated with anaphylaxis, septic or endotoxic shock, spinal cord injury, and the ingestion of vasodilating drugs. In these cases, peripheral perfusion is usually maintained and the extremities remain warm.[33] [34]
Cardiogenic shock is a relatively uncommon cause of circulatory
failure in pediatric patients when compared with its incidence in adults. In the
neonate, the usual cause is congenital heart disease with outflow obstruction or
systemic-to-pulmonary shunting, although viral or bacterial sepsis is also a common
cause. In any child, extrinsic inflow or outflow obstruction associated with tension
pneumothorax, hemopericardium, or pneumopericardium,
| Low Central Venous Pressure: Intravascular Hypovolemia | ||
|---|---|---|
| Vasoconstriction: Loss of Volume | Vasodilation: Loss of Resistance | Normal or High Central Venous Pressure: Cardiogenic |
| Blood loss | Anaphylaxis | Pump failure |
| Plasma loss | Sepsis | Shunting |
| Extracellular fluid loss | Drug intoxication | Outflow, inflow obstruction |
|
|
|
Arrhythmias |
| Modified from Crone RK: Acute circulatory failure in children. Pediatr Clin North Am 27:525, 1980. | ||
The cause and functional physiologic features of shock can frequently be identified from the history and physical examination. Specific therapy may differ in various clinical conditions, but immediate therapy should include assessment of the airway, administration of oxygen, establishment of adequate ventilation, and procurement of vascular access. A peripheral venous site may be cannulated percutaneously, but impaired perfusion may reduce the possibility of success. The intraosseous (IO) route for fluid and drug bolus or infusion is now routine in emergency settings. [39] A stiff, short-beveled trocar needle is placed into the proximal third of the tibia ( Fig. 76-1 ) or the distal end of the femur. Specific needles are manufactured for this purpose, but 18-gauge spinal needles and bone marrow aspirate needles also function well. Care must be taken to avoid the epiphysis. When the IO needle is properly positioned, its shaft is anchored in the cortex, with the needle tip in the marrow cavity. The cortex supports the needle solidly, blood and bone spicules can usually be aspirated, and infusion of fluids is easy without distorting the soft tissues of the leg. The IO line is then used to obtain blood samples for laboratory analysis, to deliver fluids, and to administer all resuscitation medications traditionally given intravenously. The IO line provides rapid, safe, emergency vascular access. Standard intravenous access should be achieved as soon as possible because IO placement degrades with time. Percutaneous cannulation of the jugular[40] and subclavian veins carries the risk of carotid puncture, hemothorax, pneumothorax, and perforation of the superior vena cava or right atrium. The smaller size of the child increases the risk of any vessel cannulation.
Specific therapy for circulatory failure is directed toward increasing cardiac output and normalizing peripheral
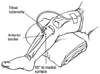 Figure 76-1
Intraosseous cannulation technique.
Figure 76-1
Intraosseous cannulation technique.
Because most children in shock are hypovolemic, augmentation of preload is the usual initial therapy. Once vascular access is obtained, blood volume should be increased with an infusion of isotonic crystalloid or colloid solution. Hypertonic resuscitation fluids have been used, but the information available is inadequate to justify their use. The amount and rate of infusion of an isotonic solution depend on the clinical condition. A good starting point is 20 mL/kg of estimated body weight, rapidly infused over a few minutes. The bolus should be repeated until improvement in perfusion is observed. If no improvement has occurred by the third bolus, ongoing losses or a different cause of shock should be suspected, and further diagnoses should be pursued.[33] [34] Intravascular volume can be assessed by physical examination, chest radiography, echocardiography, and placement of a central venous pressure (CVP) catheter.
Echocardiography has the advantage of being rapid and noninvasive, but it requires the immediate availability of sophisticated equipment and personnel skilled in interpreting pediatric echocardiograms. Structural cardiac abnormalities can be ruled out and myocardial function may be qualitatively assessed in real time. [41] Most infants and children who need ongoing assessment and cardiovascular support require a central venous catheter. Although a CVP value of 5 mm Hg is a reasonable estimate for adequate intravascular volume, there is no single optimal CVP; only with careful incremental infusions of volume (10 mL/kg per bolus) can the effect on cardiac output be assessed. Several extracardiac factors, including lung compliance, the level of positive end-expiratory pressure (PEEP), peak inspiratory pressure, and increasing abdominal pressure, affect CVP or right ventricular filling pressure; thus, each patient's optimal CVP may differ considerably. Once a reasonable CVP has been obtained, if no improvement in blood pressure, cutaneous perfusion, or urine output is observed, cardiogenic causes of circulatory failure must be considered. Arterial blood gases (ABGs), hematocrit, serum electrolytes, glucose, and calcium levels should be determined. Correction of acidosis, hypoxemia, or metabolic derangement is essential. Blood and other appropriate sites must be cultured, and broad-spectrum parenteral antibiotic coverage must be initiated if sepsis is a possibility.
Urine output can be an important indicator of the adequacy of the circulation. Urine output greater than 1 mL/kg/hr usually indicates adequate renal blood flow and cardiac output. Patients with an output less than 0.5 mL/kg/hr should be evaluated for circulatory failure, renal failure, or obstruction to urine flow.
Myocardial contractility can be improved by correcting existing metabolic derangements (hypoxia, acidosis, hypoglycemia) and by administering positive inotropic agents such as sympathomimetic amines (β-agonists), xanthines, and cardiac glycosides.
In clinical practice, the pulmonary artery catheter is used less frequently in children than in adult patients. In contrast to an adult with preexisting coronary artery disease, most conditions causing circulatory failure in children are associated with biventricular failure. In these cases, left ventricular filling pressures are reflected by right atrial pressure, and the CVP is an adequate indicator of left- and right-sided filling pressures. The pulmonary artery catheter is generally used in children for continuous measurement of pulmonary artery pressure or for intermittent measurement of cardiac output by the thermodilution technique.[42] [43] [44]
Pulmonary artery catheters are available in two sizes: the "adult" 7 French and the "pediatric" 5 French. The CVP port is 30 cm and 15 cm proximal to the tip in the 7- and 5-French catheters, respectively. Children who weigh less than 8 to 10 kg are too small for even the 5-French catheter.
Afterload reduction may be specifically indicated when "pump failure" coexists with an elevated SVR.[45] Under these circumstances, there may be an advantage to reducing afterload and ventricular work so that the ventricle can pump more efficiently with an increase in stroke volume. Afterload reduction may be effected with a direct vasodilator such as sodium nitroprusside, a β-agonist (isoproterenol), a phosphodiesterase III inhibitor (milrinone), or an α-antagonist (phentolamine or tolazoline). Afterload reduction is frequently unpredictable, and the associated hypotension may reduce coronary perfusion to such a degree that it reduces cardiac efficiency rather than improves it. Vasodilators should be used with extreme caution, and vasoconstricting agents (phenylephrine or norepinephrine) should be available for immediate use.
Pharmacologic vasoconstriction is instituted when hypotension results from an inappropriately low SVR such as in anaphylaxis and warm septic shock. [33] The major complication of this therapy is that vasoconstricting drugs produce an increase in myocardial work and oxygen consumption, with the result that any primary myocardial decompensation may be worsened.
Pharmacologic support of the circulation includes positive inotropic and chronotropic agents, vasoconstrictors and vasodilators (afterload reduction), and antiarrhythmics (see Chapter 16 and Chapter 60 ). Most currently used drugs have not been adequately studied in children, so dosage recommendations and anticipated effects are extrapolated from studies in adults, as well as from anecdotal clinical experience in children.
Positive inotropic drugs are frequently used in children to augment cardiac output in a variety of situations associated with circulatory failure. Most inotropic agents also affect the heart rate and vasomotor tone. Tachycardia is often a deleterious side effect in an adult with limited myocardial oxygen reserve, whereas in a child, an increase in heart rate is usually well tolerated and is
Commonly used inotropes are listed in Table 76-4 . Brief comments regarding their use in pediatric intensive care are provided.
Epinephrine is primarily used for children with cardiac arrest or profound myocardial depression and hypotension. Epinephrine is preferably used for initial stabilization, with the hope of switching to another inotrope with less peripheral vasoconstriction as soon as possible.[46] [47]
Dopamine is the most commonly infused inotrope in pediatrics. Its physiologic effects are very dose dependent, with dopaminergic activity in low doses, β-adrenergic activity in intermediate doses, and some α-adrenergic activity in higher doses. Specific doses can be patient dependent, but as a rule, young children require higher doses than adults do to produce the same effect.[48] In one study, an infusion of 15 µg/kg/min was needed to increase cardiac output higher than control in a group of infants after cardiac surgery.[49] This finding may reflect the decreased releasable stores of norepinephrine in the myocardium of immature ventricles.[50]
| Drug | Effect | Dose * | Inotropy | Chronotropy | Vasodilation | Vasoconstriction |
|---|---|---|---|---|---|---|
| Epinephrine (Adrenalin) | α, β | 0.05–2.0 | ++ | ++ |
|
++ |
| Isoproterenol (Isuprel) | β (1, 2) | 0.05–2.0 | ++ | ++ | + |
|
| Dopamine (Inotropin) | δ | 1–3 |
|
|
+Renal splanchnic |
|
|
|
β > α | 5–15 | + | + |
|
+/- |
|
|
β, α | >15 | + | + |
|
+ |
| Milrinone |
|
Bolus: 50 µg/kg over 10 min | + |
|
+ |
|
|
|
|
Infusion: 0.375–0.75 |
|
|
|
|
| Norepinephrine | α ≫ | 0.05–1.0 | sl+ | + |
|
++ |
|
|
β |
|
|
|
|
|
| Nitroprusside |
|
0.5–10 |
|
|
++ |
|
|
|
|
|
|
|
Art > venous |
|
| Nitroglycerin |
|
1–20 |
|
|
++ |
|
Isoproterenol is a pure β-agonist medication with very strong chronotropic effects that are often better tolerated in children than adults. Even though isoproterenol is a strong inotrope, it causes vasodilation, which may be problematic in a patient who is volume depleted.
Dobutamine was designed to provide positive inotropy and afterload reduction, without the chronotropy of isoproterenol. However, clinical experience with this drug in children has demonstrated an associated tachycardia.[51] [52]
Norepinephrine, an α- and β-agonist drug, is rarely used in children except in situations of near-normal cardiac function and extreme peripheral vasodilation. Examples are warm septic shock, anaphylaxis, and sympathetic blocks associated with regional anesthesia.[46] [47]
Milrinone is a noncatecholamine, nonglycoside inotrope and vasodilator. A phosphodiesterase III inhibitor, it increases cyclic adenosine monophosphate. It is the only inotrope that has undergone a randomized clinical trial proving its effectiveness in the outcome of children undergoing cardiac surgery with low cardiac output syndrome.[53] Milrinone is administered as a loading dose of 25 to 75 µg/kg over a period of 10 minutes and a maintenance infusion of 0.25 to 0.75 µg/kg/min. Medication risks include hypotension and tachycardia, which occur primarily with the loading bolus and are usually responsive to fluid infusion. Renal failure significantly increases the terminal elimination half-life of this drug.[54] [55] [56]
Digitalis is an excellent medication for the long-term treatment of myocardial failure in children. Its long half-life
Calcium is an often overlooked inotrope. When serum ionized calcium levels are low, administration of calcium produces a positive inotropic effect; if ionized calcium levels are normal, however, the inotropic effect is much less marked. Low ionized calcium levels are most commonly documented in patients with DiGeorge's syndrome or septic shock, after volume replacement with citrated blood products, and in neonates with relatively unstable calcium metabolism.[58] [59] Calcium can also have a number of effects on the conduction system. In children with a normal ionized calcium level, rapid administration of calcium through a central venous catheter can cause severe bradycardia or asystole. This effect can be exaggerated in the presence of hypokalemia and digitalis. There have also been reports of calcium precipitating malignant ventricular arrhythmias in adult patients with coronary artery disease; the relative absence of coronary artery disease in children minimizes this complication in the pediatric population. The vasomotor effects of calcium are somewhat controversial, but most reports show an increase in SVR. Increased PVR has also been documented.[60]
Acidosis profoundly depresses myocardial function and is a sensitive indicator of inadequate tissue perfusion. Initial correction with 1 to 2 mEq/kg of sodium bicarbonate is indicated for a pH of less than 7.20 after establishment of adequate ventilation (PCO2 <40 mm Hg). The circulatory system is refractory to sympathomimetic amines in the presence of severe acidosis; thus, inotropic agents, even in massive doses, are rendered ineffective unless the acidosis is corrected. After initial correction of pH, persistence or reappearance of metabolic acidosis suggests a return to an underperfused state and is cause for immediate alarm and therapeutic action. Sodium bicarbonate therapy can be used only as a stopgap measure to render the circulation amenable to pharmacologic support. Repeat infusions of sodium bicarbonate rapidly lead to hypernatremia and hyperosmolarity and cannot neutralize the ongoing lactic acidosis associated with inadequate tissue perfusion.
Vasodilators are used in children for four main purposes: (1) to control systemic hypertension, (2) to increase cardiac output by decreasing afterload, (3) to control pulmonary hypertension, and (4) to attempt to control cardiac shunting. The use of vasodilators for control of systemic hypertension and for increasing cardiac output in children with CHF has been quite successful. By contrast, treatment of pulmonary hypertension and cardiac shunting with vasodilators has shown limited results. The primary problem is that most available vasodilators work on the systemic circulation as well as the pulmonary circulation. Systemic hypotension and increases in left-to-right shunting have resulted from attempts to treat pulmonary hypertension with vasodilators. Inhaled NO has demonstrated some efficacy as a selective pulmonary vasodilator in the treatment of pulmonary hypertension.
This vasodilator relaxes arteriolar and venous smooth muscle and thereby produces a decrease in afterload and possibly a decrease in preload. The half-life is only minutes; as such, it is very safe to titrate infusions of this drug to a desired effect. Most common indications for its use are to control severe systemic hypertension, precipitate an intraoperative episode of controlled hypotension in an attempt to decrease blood loss, and increase cardiac output in children with low cardiac output syndromes (myocarditis, post-cardiac surgical status).[61] Sodium nitroprusside can be used for days without problems. Potential difficulties include cyanide and thiocyanate poisoning. Cyanate is an intermediate metabolite of nitroprusside; thiocyanate is the final metabolite and is slowly cleared by renal excretion. Thiocyanate toxicity is more common than cyanate toxicity. Thiocyanate levels of 10 mg/dL are associated with weakness, hypoxia, nausea, muscle spasms, and disorientation. Treatment involves discontinuing the administration of nitroprusside. [46] [62]
Intravenous nitroglycerin is similar in effect to sodium nitroprusside, except that nitroglycerin has a relatively stronger vasodilating effect on the venous capacitance system than on the arterial bed.[63]
This vasodilator is routinely used to control systemic hypertension. Hydralazine produces vascular smooth muscle relaxation in the arterial system much more than in the venous system. Certain unpleasant effects of hydralazine therapy (i.e., headache, nausea, dizziness, sweating, tremors) have been described. The most important acute side effect is the cardiovascular effect of reflex tachycardia, with possible increased cardiac output; a β-antagonist (i.e., labetalol) is often used as adjunctive therapy to counteract this effect.[64] [65]
These competitive α-adrenergic blockers have been used in the treatment of pulmonary hypertension. The success of this therapy is not uniform, and these drugs are now rarely used.[66] They are most commonly used in the preoperative treatment of pheochromocytoma. Serious side effects of these drugs include tachycardia, ventricular arrhythmias, hypotension, and tissue edema.
Though classified as a vasodilator, PGE1 is a unique drug that has greatly improved the care of neonates with heart disease. PGE1 acts directly on vascular smooth muscle; when infused at a rate of 0.1 µg/kg/min, it maintains patency of the ductus arteriosus or even reopens the ductus in neonates. This response is dependent on such factors as age and the state of contraction of the ductus. Side effects of apnea, hypotension from systemic vasodilation, or central nervous system (CNS) excitability should be anticipated. This drug is indispensable in patients with ductus-dependent cardiac lesions such as interrupted aortic arch, critical aortic stenosis, or hypoplastic left heart syndrome, in which systemic flow is supplied through the ductus.[31] It is equally indispensable for pulmonary atresia and
With the discovery of NO as one of the endothelium-derived relaxation factors, we now have a drug that is capable of selectively vasodilating the pulmonary vasculature.[67] NO can be administered by inhalation to patients with pulmonary hypertension, in whom it reduces PVR.[68] It diffuses across the alveoli, dilates the pulmonary vascular bed, and is inactivated by binding with hemoglobin before delivery to the systemic vasculature. NO does not appear to cause systemic vasodilation or clinically significant methemoglobinemia in the doses administered (5 to 80 ppm).[69] Studies have shown it to be effective in reducing PVR and improving outcome in neonates with reactive pulmonary hypertension.[70]
Arrhythmias are less common in children than adults, most likely because of the relative lack of atherosclerosis in the pediatric age group (see Chapter 34 and Chapter 51 ). However, a few categories of arrhythmias should be considered.
Perhaps the most common pediatric arrhythmia is sinus bradycardia caused by hypoxemia. Hence, the primary antiarrhythmic of childhood is oxygen. Heart block is seen in children after surgical repair of congenital heart disease. If the block persists for more than 2 weeks postoperatively, a pacemaker is generally indicated.[71] Another cause of heart block is connective tissue disease; in fact, in infants born to mothers with systemic lupus erythematosus, heart block has been diagnosed in utero.[72]
Sick sinus syndrome is usually associated with surgery, myocarditis, or myocardiopathy.[73] Prolonged QT syndrome, an abnormality in ventricular repolarization, is a familial disorder that can result in sudden cardiac death. Other than sinus bradycardia, the most common arrhythmia is supraventricular tachycardia (SVT), a narrow QRS complex with a regular R-R interval. In infants and neonates, the rate can be 200 to 300 beats/min, and in older children, 150 to 250 beats/min. SVT causes slow myocardial decompensation. An older patient with SVT will frequently complain of palpitations or a racing heart rate and seek medical care long before CHF ensues. By contrast, a younger child is unable to verbalize the sensation of palpitations and will most likely present with CHF. Treatment includes vagal maneuvers, adenosine (0.1 to 0.2 mg/kg, with a maximum single dose of 12 mg) by rapid intravenous bolus, and direct current cardioversion (0.25 to 1 J/kg).[74] [75]
Essential hypertension is not a common problem in children. When
hypertension does occur, it is secondary ( Table
76-5
) and often more difficult to control. The acute onset of severe systemic
arterial hypertension is a medical emergency because of the potential for cardiovascular
decompensation, as well as the CNS complications of encephalopathy, seizures, and
intracranial hemorrhage. In older children, the neurologic manifestations of
| Renal |
| Acute glomerulonephritis (e.g., poststreptococcal, Henoch-Schönlein purpura) |
| Hemolytic-uremic syndrome |
| Chronic glomerulonephritis (all types) |
| Acute and chronic pyelonephritis |
| Congenital malformations (dysplasia, hypoplasia, cystic diseases) |
| Tumors (e.g., Wilms', leukemic infiltrate) |
| Post-renal transplantation status; also rejection |
| Oliguric renal failure |
| Trauma |
| Obstructive uropathy |
| After genitourinary surgery |
| Blood transfusions in children with azotemia |
| Cardiovascular |
| Coarctation of the aorta |
| Renal artery abnormalities (e.g., stenosis, thrombosis) |
| Takayasu's disease |
| Endocrine |
| Pheochromocytoma |
| Neuroblastoma |
| Adrenogenital disease |
| Cushing's syndrome |
| Hyperaldosteronism |
| Hyperthyroidism |
| Hyperparathyroidism |
| Iatrogenic |
| Intravascular volume overload |
| Sympathomimetic administration (e.g., epinephrine, ephedrine) |
| Corticosteroid administration |
| Rapid intravenous infusion of methyldopa |
| Miscellaneous |
| Immobilization (e.g., fractures, burns, Guillain-Barré syndrome) |
| Hypercalcemia (e.g., hypervitaminosis D, metastatic disease, sarcoidosis, some immobilized patients) |
| Hypernatremia |
| Stevens-Johnson syndrome |
| Increased intracranial pressure (any cause) |
| Dysautonomia |
| After resuscitation |
Classes of antihypertensive drugs are listed in Table 76-7 . In the intensive care unit (ICU), moderate hypertension often accompanies and complicates other disease processes; therefore, familiarity with all these drugs is necessary. However, if malignant hypertension is the admitting diagnosis, an aggressive approach is mandated
| Converting enzyme inhibitors to decrease angiotensin II |
| Caution in the face of renovascular disease |
| Captopril |
| Enalapril |
| Calcium channel blockers, usually type II blockers |
| Caution: Myocardial decompensation |
| Nifedipine |
| Nimodipine |
| Diuretics, usually as an adjunct |
| β-Adrenergic blockers |
| Caution: Reactive airway disease |
| Propranolol |
| Esmolol |
| α2 -Antagonist |
| Prazosin |
| Central α-agonist |
| Clonidine |
| α- and β-adrenergic blocker |
| Labetalol |
| Dilators |
| Hydralazine |
| Diazoxide |
|
|
|
|
|
|
|
|
|
|
|
|
|