|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A survey mailed to 56 anesthesiologists revealed that the end points deemed to be most important to patients were postoperative incisional pain, nausea, vomiting, preoperative anxiety, and discomfort from establishment of intravenous access, in that order.[123] Patients concurred. Patients rated the following as most undesirable (in order): vomiting, gagging on the tracheal tube, incisional pain, nausea, recall without pain, residual weakness, shivering, sore throat, and somnolence.[124]
Thus, a key part of recovery is adequate control of pain during rest (rest pain) and pain with activity (incident pain). Rest pain is generally easier to alleviate. Incident pain is more difficult to manage. The choice of a particular postoperative pain management regimen depends on the anticipated pain intensity and the capacity for postoperative monitoring and supervision.
To further evaluate patient perceptions regarding common side
effects of anesthesia, 66 patients were surveyed to determine how much money they
were willing to pay
| Outcome | Median Dollar Amount |
|---|---|
| Pain | 110 |
| Vomiting | 100 |
| Nausea | 100 |
| Difficulty voiding | 60 |
| Hangover | 50 |
| Shivering | 50 |
| Feeling cold | 30 |
| Sore throat | 15 |
These data are presented to further highlight how highly patients do, in fact, desire to avoid pain. However, anesthesiologists are unable to predict which specific outcome is of highest importance to a particular surgical patient. [126] Because it is difficult to know a priori for any given patient which clinical anesthesia outcomes (avoiding pain or avoiding nausea as a first priority) are of greatest concern, it may be useful to actively engage patients (as part of the preoperative evaluation and informed consent process) to identify, for example, their three most important clinical outcomes and design the anesthetic to optimize these outcomes. For example, if a patient's highest concern is to avoid nausea after the anesthetic, to improve clinical quality the anesthetic plan should be constructed with that aim.
Despite new techniques and increased emphasis on relieving acute pain, postoperative pain remains undertreated. Reasons include confusion about who is responsible for analgesia, providers' lack of knowledge regarding the effective dose ranges and duration of action of opioids, and fears of respiratory depression and addiction.[127] [128] [129] A questionnaire survey study of 49 nurses working in surgical units in Denmark found that[130]
Many factors influence the onset, incidence, and severity of postoperative pain. The very young and very old experience less pain than do people in the middle years of life. Preoperative neurotic personality traits tend to increase postoperative pain, as does the fear of pain itself. The way in which a patient is prepared for the experience of pain preoperatively can markedly alter the perception of post-operative pain ( Fig. 71-13 ).[132]
The site of the operation certainly influences the severity of pain. In general, thoracotomy appears to be the most
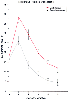 Figure 71-13
Ninety-seven patients undergoing abdominal surgery were
divided into two groups. The control group had a preoperative visit in which the
anesthetist discussed the patient's medical condition but did not explain any postoperative
events. The special care group had a careful preoperative explanation about postoperative
pain, including its character, intensity, and management, and stressed that it was
a normal occurrence. The special care group required significantly less morphine
for pain relief than the control group did, thus emphasizing the importance of the
preoperative visit in postoperative care. (Redrawn from Egbert LD, Battit
GE, Welsch CE, et al: Reduction of postoperative pain by encouragement and instruction
of patients. N Engl J Med 270:825, 1964.)
Figure 71-13
Ninety-seven patients undergoing abdominal surgery were
divided into two groups. The control group had a preoperative visit in which the
anesthetist discussed the patient's medical condition but did not explain any postoperative
events. The special care group had a careful preoperative explanation about postoperative
pain, including its character, intensity, and management, and stressed that it was
a normal occurrence. The special care group required significantly less morphine
for pain relief than the control group did, thus emphasizing the importance of the
preoperative visit in postoperative care. (Redrawn from Egbert LD, Battit
GE, Welsch CE, et al: Reduction of postoperative pain by encouragement and instruction
of patients. N Engl J Med 270:825, 1964.)
The use of morphine by titration is often the first step in postoperative pain management. Ethnicity (white race), emergency surgery, major surgery, surgery exceeding 100 minutes, and pain score on arrival in the PACU are factors predictive of increased morphine requirements.[134] Intravenous morphine titration every 5 minutes with an unlimited number of boluses and early subcutaneous administration provided the best analgesic regimen in a study investigating different methods of titration.[135]
Administering morphine at the end of surgery (1 to 3 mg intravenously every 5 or 10 minutes) instead of waiting until the patient is in the PACU improves pain relief with less respiratory depression.[136] The presence of pain does not prevent narcotic-induced respiratory depression.[137] Patients will need encouragement to cough and breathe deeply.
Patient-controlled analgesia permits the patient to determine the timing of analgesic doses and allows for improved titration of analgesia. It also minimizes patient anxiety. Patients receiving this form of pain therapy should have it begun in the PACU.[138] Although morphine has been the gold standard for this form of therapy, some institutions have eliminated meperidine patient-controlled analgesia because of reports of central nervous system toxicity and seizures in patients receiving high doses.
Nonsteroidal anti-inflammatory drugs (NSAIDs) can be part of an effective multimodal analgesia protocol that includes instructing the patient to take pain medication as soon as discomfort occurs. NSAIDs are useful for postoperative pain management because surgery causes both pain and inflammation. NSAIDs may be divided into three groups: NSAIDs with predominant analgesic effect (ketorolac, naproxen), NSAIDs that are essentially anti-inflammatory (oxicams), and NSAIDs that have both analgesic and anti-inflammatory effects (diclofenac, ketoprofen, indomethacin). [139]
NSAIDs, including ketorolac, diclofenac, naproxen, flurbiprofen, ketoprofen, diflunisal, and ibuprofen, do not have a preemptive analgesic effect if administered before incision.[140]
Ketorolac, though not as potent as the narcotics, can be an effective alternative to narcotic analgesics in the PACU. Depending on the type of surgery, adding ketorolac reduces the total opioid dose by a third (with a range from 0% to 73%, depending on the type of surgery) and improves the amount of pain relief.[141] However, a concomitant reduction in opioid side effects (e.g., nausea, vomiting) is more difficult to document because studies have insufficient numbers of patients enrolled to detect differences in the incidence of opioid-related side effects.
The risk for adverse events with ketorolac increases with high doses, with prolonged therapy (>5 days), or in vulnerable patients (e.g., the elderly). The incidence of serious adverse events has declined since dosage guidelines were revised.
Ketorolac is as safe as ketoprofen and diclofenac for the treatment of pain after major surgery. To evaluate the risk of serious adverse effects with ketorolac (versus diclofenac or ketoprofen) in adult patients after elective major surgery, a total of 11,245 patients at 49 European hospitals (in eight countries) were studied.[142] The doses actually administered varied by country. For example, the parenteral median ketorolac dose varied from 40 to 100 mg.
Overall, 155 patients (1.38%) had a serious adverse outcome. Nineteen deaths (0.17%) occurred, none of which were related to the use of NSAIDs. The most frequent adverse outcome was surgical-site bleeding in 117 patients (1.04%), 61 of whom received ketorolac and 56 received one of the comparators.
Twelve patients had allergic reactions (0.11%), 10 patients had acute renal failure (0.09%), and 4 suffered gastrointestinal bleeding (0.04%). When a postoperative anticoagulant was administered, patients who received ketorolac were equally likely to have surgical-site bleeding. The introduction of oral[143] [144] [145] and intravenous cyclooxygenase-2[146] [147] selective NSAIDs may add another analgesic type for use in the PACU.
The use of narcotics in the epidural space to control postoperative pain is a very effective approach (also see Chapter 72 ). Morphine, 2 to 4 mg diluted to 10 mL, provides prompt analgesia with a duration of action of about 12 hours. Complications include respiratory depression, which is dose related and can occur as long as 6 hours after injection of the morphine. Significant respiratory depression occurs in less than 1% of patient receiving epidural narcotics and can be reversed with naloxone.
Synthetic narcotics have also been used successfully for epidural analgesia.[148] [149] About 15% to 20% of patients complain of pruritus. Nausea and urinary retention have also been reported to be complications. The technique is most helpful when used for patients undergoing major thoracic or abdominal surgery who are at high risk for complications of parenteral analgesic therapy.[150] [151] [152] [153] [154]
Regional anesthesia has been used for the relief of postoperative pain to avoid narcotic-induced respiratory depression (also see Chapter 42 Chapter 43 Chapter 44 Chapter 45 and Chapter 72 ). Instillation of local anesthetic into a wound can be very efficacious and is simple to perform. [155]
Continuous epidural blockade can provide good postoperative analgesia and, when done in the thoracic space, can permit early postoperative ambulation (also see Chapter 72 ).[156] The use of epidural analgesia probably results in somewhat better arterial oxygenation; however, the incidence of postoperative respiratory complications appears to be no better than with narcotics.[157] Patients receiving epidural pain relief can be ambulated earlier, thereby permitting earlier hospital discharge.[158]
The use of regional anesthesia for postoperative pain relief appears to be best suited for patients with preexisting lung disease in whom narcotics would be hazardous and when a regional technique could relieve pain without adversely affecting respiration.[159]
|
|
|
|
|
|
|
|
|
|
|
|
|