|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Approximately 140 million people worldwide live permanently at altitudes higher than 2500 m,[205] and possibly an equal number visit high altitudes each year, many of whom will require medical care. There is therefore clearly a need for anesthesia and critical care practitioners to understand the physiology of this environment.
Unlike the relationship between ambient pressure and water depth, the relationship between pressure and altitude is a nonlinear one (see Fig. 70-2 ). The physiologic effects of altitude exposure are mainly attributable to a reduction in the following three variables: inspired PO2 , ambient pressure, and gas density. Extreme or prolonged exposure to high altitude may be accompanied by additional factors that may modulate physiologic responses, such as hypothermia, exertion, dehydration, sunburn, and polycythemia. Pregnant women and individuals with hypoxic conditions such as cerebrovascular disease, congenital heart defects, coronary artery disease, and hypoxic lung disease may carry additional risks that require medical evaluation. These issues have been extensively discussed by Hackett.[206]
O2
partial pressures at an altitude of 4572 m are shown
in Table 70-5
. The major
mechanism resulting in hypoxia at altitude is a reduction in inspired PO2
.
However, an additional reason for arterial hypoxemia is failure of erythrocytes
in the pulmonary circulation to fully equilibrate with alveolar gas (diffusion nonequilibrium).
[207]
This failure to equilibrate is especially
likely to be important during exercise, when increased pulmonary blood flow reduces
capillary transit time. In addition, the ventilation-perfusion (V̇A/![]() )
mismatch is increased, which is believed to be related to pulmonary hypertension.
[208]
)
mismatch is increased, which is believed to be related to pulmonary hypertension.
[208]
The effects of acute and chronic hypoxia on normal physiology have been reviewed in a monograph.[209] Responses to acute hypoxia include an increase in heart rate and cardiac output. There is a corresponding increase in organ blood flow, including cerebral blood flow. Hypoxia also induces hyperpnea (because of an increased breathing rate), to a degree that varies considerably among individuals. End-tidal PCO2 measured in one subject breathing air at the summit of Mt. Everest (barometric pressure of 263 mm Hg) was 7.5 mm Hg, whereas PAO2 was 37.6 mm Hg.[210] This hypocapnia has several effects. It results in an increased affinity of Hb for O2 (leftward shift of the Hb-O2 dissociation curve), which enhances oxygenation of blood at the lung while simultaneously interfering with transport of O2 from tissue capillary to mitochondrion. The overall effect on O2 transport is beneficial, however.[211]
Hypoxemia may be particularly accentuated during sleep. Figure 70-15 depicts continuous pulse oximetry during the transition from sleep to wakefulness at a simulated altitude of 4572 m in a hypobaric chamber; periodic breathing and cyclic oscillation of SpO2 were observed during sleep but normalized on awakening.
Acute exposure to altitudes in the range of 4000 to 5000 m causes a decrease in arterial PaO2 to approximately 40 mm Hg and a decrease in arterial Hb O2 saturation to approximately 75%. Although colors may be perceived as somewhat less bright than normal, other than limitations in exercise capacity at these altitudes, there
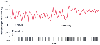 Figure 70-15
Pulse oximetry and breathing pattern (spikes at bottom
representing exhalations) are shown in this subject in a hypobaric chamber at a simulated
altitude of 4572 m. During sleep, periodic breathing and oscillatory alterations
in SpO2
change after waking up to a more
regular breathing pattern and a steady SpO2
.
Figure 70-15
Pulse oximetry and breathing pattern (spikes at bottom
representing exhalations) are shown in this subject in a hypobaric chamber at a simulated
altitude of 4572 m. During sleep, periodic breathing and oscillatory alterations
in SpO2
change after waking up to a more
regular breathing pattern and a steady SpO2
.
Severe hypoxia can cause loss of consciousness. One measure of performance during hypoxia that is used in aviation physiology is the effective performance time, defined as the amount of time that the individual is able to perform useful flying duties.[213] At an altitude of 5500 m, the effective performance time is 20 to 30 minutes; it is reduced to 2.5 to 3 minutes at 8500 m and 1 to 2 minutes at 9100 m.
Gradual exposure to altitude or chronic exposure results in a series of adaptive changes that allow individuals to function extremely well and do substantial physical work at altitudes at which newcomers can barely function. For instance, Mt. Everest has been climbed without the benefit of supplemental O2 , whereas acute exposure to such an altitude would result in rapid loss of consciousness. Subjects exposed to a progressively lower barometric pressure, up to a simulated altitude of 8848 m in a hypobaric chamber over the course of 40 days, were able to work on an exercise ergometer at 120 W while their mean PaO2 was only 30 mm Hg (arterial Hb O2 saturation of 58%).[214] In the same study, the blood Hb concentration increased from 13.5 to 17.0 g/dL (hematocrit from 40.4% to 51.9%). The development of polycythemia is perhaps one mechanism by which tolerance to hypoxia occurs. However, the mechanisms of adaptation are still incompletely understood.
One of the adaptive mechanisms is a gradual reduction in plasma bicarbonate,[214] preceded by a decrease in cere-brospinal fluid (CSF) bicarbonate,[215] which tends to offset the initial respiratory alkalosis. After a 40-day simulated "climb" of Mt. Everest in a hypobaric chamber, resting serum bicarbonate had decreased from 22.2 to 9.9 mM, whereas pH had increased from 7.43 to 7.56.[214] Other adaptive changes include increases in hematocrit and capillary density, as well as undoubtedly other mechanisms as yet undiscovered.
Despite the adaptive mechanisms, careful neuropsychological testing of subjects before and after mountaineering expeditions to extreme altitudes of 7000 m or greater has shown evidence of mild persistent impairment in performance.[216] [217] Although it is not certain that these abnormalities on formal testing are of any functional significance, investigators have reported that impairments may last up to several months after exposure. Individuals who have the most vigorous hypoxic ventilatory response and who therefore hyperventilate the most usually tolerate altitude exposure the best. However, Hornbein and associates [216] have shown that neuropsychological impairment after prolonged exposure to extreme altitude is correlated with a brisk hypoxic ventilatory response. These authors hypothesized that despite a higher PaO2 in individuals with a vigorous hypoxic ventilatory response, brain O2 delivery may be lower because of hypocapnic cerebral vasoconstriction. Support for this hypothesis is provided by a study in which hypocapnia was shown to result in a reduction in brain blood volume and oxidized cytochrome a,a3 .[218]
Data on the fetal effects of maternal hypoxia are sparse. However, if changes in fetal heart rate (FHR) are used as an end point, the fetus appears to be relatively resistant to moderate degrees of maternal hypoxia. Administration of 15% O2 (inspired PO2 of 108 mm Hg, equivalent to an altitude of 2500 m) for 4 minutes to five primigravid women at 36 to 38 weeks' gestation resulted in an increase in FHR of only 2 to 3 beats per minute.[219] The same investigator observed no change in FHR after the administration of mixtures as low as 10% O2 (inspired PO2 of 71 mm Hg, equivalent to an altitude of 5300 m) for 20 minutes in 8 of 28 pregnant women. Polvi and colleagues [220] administered 10% O2 for 10 minutes to pregnant women at 35 to 41 weeks' gestation. FHR, heart rate variability, and Doppler velocimetry in the umbilical and middle cerebral arteries of the fetus were unchanged from baseline. Acute hypoxia of this magnitude appears to exert no detectable effects on a healthy fetus because of augmentation of uterine blood flow. It is therefore unlikely that acute exposure
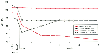 Figure 70-16
Pulse oximetry in infants born at various altitudes.
In infants born in Lhasa (altitude of 3658 m), Han Chinese children have progressive
arterial desaturation, whereas Tibetan infants, following a slight drop shortly after
birth, have a stable SpO2
. (Redrawn
from Niermeyer S: Cardiopulmonary transition in the high altitude infant. High
Alt Med Biol 4:225, 2003.)
Figure 70-16
Pulse oximetry in infants born at various altitudes.
In infants born in Lhasa (altitude of 3658 m), Han Chinese children have progressive
arterial desaturation, whereas Tibetan infants, following a slight drop shortly after
birth, have a stable SpO2
. (Redrawn
from Niermeyer S: Cardiopulmonary transition in the high altitude infant. High
Alt Med Biol 4:225, 2003.)
Additional adaptations to chronic exposure to high altitude specific to pregnant women include an increase in placental capillary volume and a reduction in villous membrane thickness.[221] Babies born at altitude, even as high as 4329 m, have scalp vein O2 tensions similar to those obtained from babies born at sea level.[222] Consequently, a significant reduction in maternal O2 delivery can be tolerated without any impact on fetal O2 consumption.
As with adults, SpO2 decreases with increasing altitude, but the decrease also depends on activity (lower during sleep), postnatal age ( Fig. 70-16 ), and race.[223] In Lhasa, Tibet (altitude, 3658 m), Chinese Han infants tend to have lower SpO2 than native Tibetan infants do. In the former, SpO2 during sleep progressively decreases from 90% shortly after birth to around 76% at 4 months; in the latter, SpO2 decreases slightly during the first week of life to around 86% and then remains fairly constant (see Fig. 70-16 ). Perinatal hypoxia appears to predispose to a blunted hypoxic ventilatory drive in adulthood. [224]
At sea level, PA pressure decreases to normal levels within 24 hours after birth, whereas at altitude, PA pressure often remains elevated for several weeks or throughout infancy.[223] In a series of 35 consecutive infants born at term in Leadville, Colorado (altitude, 3179 m), during the first 3 months of life 17% experienced elevated PA pressure or respiratory distress requiring supplemental O2 or mechanical ventilation (or both).[223] A patent foramen ovale was present at 6 months in 7 of 16 infants born in La Paz, Bolivia (altitude, 3700 to 4000 m).[225]
Respiratory infections in children at altitude are commonly associated with severe hypoxemia. Moreover, hypoxemia in children with symptoms of respiratory infection at altitude is both sensitive and specific for the diagnosis of pneumonia. [223] Perinatal pulmonary hypertension seems to predispose to exaggerated hypoxic pulmonary vasoconstriction in adulthood.[226]
|
|
|
|
|
|
|
|
|
|
|
|
|