Increased Partial Pressure of Oxygen
Breathing O2
at increased ambient pressure will lead
to elevation of alveolar O2
tension (PAO2
),
which can be calculated according to the alveolar gas equation for O2
:
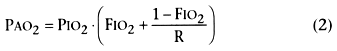
where PIO2
and FIO2
are the inspired partial pressure and fractional O2
concentration, respectively;
PACO2
is alveolar PCO2
,
assumed to equal arterial PCO2
(PaCO2
);
and R is the respiratory exchange ratio (usually ≅0.8 at rest). Calculated values
are shown in Table 70-4
.
Arterial PO2
(PaO2
)
has
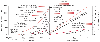 Figure 70-2
Ambient pressure as a function of altitude and water
depth. Whereas ambient pressure increases linearly with depth, pressure and altitude
are not linearly related. As air is inspired and humidified, there is a small and
usually insignificant drop from atmospheric PO2
to inspired PO2
. At altitude, however,
this decrease accounts for a greater proportion of the total ambient pressure. The
O2
partial pressure line in the water is shown for a constant FIO2
of 21%. At increasing depth, the inspired PO2
eventually exceeds the pulmonary toxic limit (≅14 m in depth) and the central
nervous system toxic limit (≅70 m in depth). The threshold for high-pressure
nervous syndrome and pressure reversal of anesthesia (observed in non-narcotic atmospheres)
is around 150 to 200 m in depth. The shaded red bars
represent the depth or altitude ranges over which risk progresses from low (light
shading) to high (dark shading). AMS,
acute mountain sickness; HACE, high-altitude cerebral edema; HAPE, high-altitude
pulmonary edema.
Figure 70-2
Ambient pressure as a function of altitude and water
depth. Whereas ambient pressure increases linearly with depth, pressure and altitude
are not linearly related. As air is inspired and humidified, there is a small and
usually insignificant drop from atmospheric PO2
to inspired PO2
. At altitude, however,
this decrease accounts for a greater proportion of the total ambient pressure. The
O2
partial pressure line in the water is shown for a constant FIO2
of 21%. At increasing depth, the inspired PO2
eventually exceeds the pulmonary toxic limit (≅14 m in depth) and the central
nervous system toxic limit (≅70 m in depth). The threshold for high-pressure
nervous syndrome and pressure reversal of anesthesia (observed in non-narcotic atmospheres)
is around 150 to 200 m in depth. The shaded red bars
represent the depth or altitude ranges over which risk progresses from low (light
shading) to high (dark shading). AMS,
acute mountain sickness; HACE, high-altitude cerebral edema; HAPE, high-altitude
pulmonary edema.
been estimated from calculated values of PAO2
,
assuming that the arterial-alveolar PO2
ratio remains constant.[64]
Whereas at 1 ATA the
fraction of O2
in arterial blood that is carried dissolved in plasma is
minimal, it can be seen that at elevated PaO2
in the range of 1000 to 2000 mm Hg, significant quantities of O2
may exist
in dissolved form ( Fig. 70-3
).
TABLE 70-3 -- Units of pressure
|
Atmospheres Absolute (ATA) |
Absolute Pressure (mm Hg) |
Gauge Pressure (mm Hg) |
Feet of Sea Water (fsw) |
Meters of Water (msw) |
|
1 |
760 |
0 |
0 |
0 |
|
2 |
1520 |
760 |
33 |
10 |
|
3 |
2280 |
1520 |
66 |
20 |
|
6 |
4560 |
3800 |
165 |
50 |
*Assuming constant
PaO2
/PAO2
ratio.[64]
Increased PaO2
has
at least four pharmacologic effects:
- Increased blood O2
content
- Vasoconstriction
- Antibacterial action, particularly against anaerobic bacteria
- Inhibition of endothelial neutrophil adhesion in injured tissue
 Figure 70-3
Blood O2
content versus PO2
.
Virtually complete saturation of hemoglobin with O2
occurs at a PO2
of 100 mm Hg. Further increases in PO2
do not alter the quantity of O2
bound to hemoglobin. However, there is
a linear increase in total blood O2
content with PO2
because of increasing quantities of O2
dissolved in plasma.
Figure 70-3
Blood O2
content versus PO2
.
Virtually complete saturation of hemoglobin with O2
occurs at a PO2
of 100 mm Hg. Further increases in PO2
do not alter the quantity of O2
bound to hemoglobin. However, there is
a linear increase in total blood O2
content with PO2
because of increasing quantities of O2
dissolved in plasma.
The increased arterial O2
content underlies the rationale
for administering HBO for the treatment of ischemic conditions, for example, ischemic,
nonhealing wounds. The elevation in PaO2
leads to an increase in tissue PO2
, which
can be estimated by using transcutaneous PO2
electrodes, even in ischemic tissue.[65]
[66]
The second effect is an explanation for the effectiveness of HBO in the treatment
of traumatic edema (e.g., crush injury). The mechanism of HBO-induced vasoconstriction
appears to be inactivation of nitric oxide (NO) as a result of increased production
of superoxide[67]
[68]
and possibly decreased release of NO from circulating S-nitrosohemoglobin.
[67]
[69]
[70]
These two effects, increased O2
content and vasoconstriction,
lead to hemodynamic changes,[70]
[71]
which are shown in Table 70-5
.
The elevation in PaO2
that occurs while
breathing 100% O2
at 3 ATA results in a drop in cardiac output and heart
rate and an increase in total peripheral resistance. There is also a slight increase
in mean arterial pressure.
The other major pharmacologic effect of increased PaO2
is the inhibition of toxin production and growth of certain anaerobic bacteria.
Additionally, increased PaO2
has been
shown to return phagocytic function and the ability of aminoglycosides to kill aerobic
bacteria in ischemic tissue to normal.[31]
[72]
HBO also has some poorly characterized microcirculatory and cellular
effects. Zamboni and colleagues[36]
[37]
[73]
described a reduction in blood flow on reperfusion
of ischemic myocutaneous tissue flaps. This decrease in flow appears to be due to
leukocyte adherence to the capillary endothelium mediated by leukocyte adhesion glycoprotein
CD18,[74]
an effect that is prevented and ameliorated
by HBO treatment.[36]
[37]
[73]
[75]
In an
animal model, timely administration of HBO also appears to decrease the lipid peroxidation
in the brain that occurs after treatment of carbon monoxide (CO) poisoning.[76]
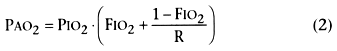
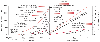 Figure 70-2
Ambient pressure as a function of altitude and water
depth. Whereas ambient pressure increases linearly with depth, pressure and altitude
are not linearly related. As air is inspired and humidified, there is a small and
usually insignificant drop from atmospheric PO2
to inspired PO2
. At altitude, however,
this decrease accounts for a greater proportion of the total ambient pressure. The
O2
partial pressure line in the water is shown for a constant FIO2
of 21%. At increasing depth, the inspired PO2
eventually exceeds the pulmonary toxic limit (≅14 m in depth) and the central
nervous system toxic limit (≅70 m in depth). The threshold for high-pressure
nervous syndrome and pressure reversal of anesthesia (observed in non-narcotic atmospheres)
is around 150 to 200 m in depth. The shaded red bars
represent the depth or altitude ranges over which risk progresses from low (light
shading) to high (dark shading). AMS,
acute mountain sickness; HACE, high-altitude cerebral edema; HAPE, high-altitude
pulmonary edema.
Figure 70-2
Ambient pressure as a function of altitude and water
depth. Whereas ambient pressure increases linearly with depth, pressure and altitude
are not linearly related. As air is inspired and humidified, there is a small and
usually insignificant drop from atmospheric PO2
to inspired PO2
. At altitude, however,
this decrease accounts for a greater proportion of the total ambient pressure. The
O2
partial pressure line in the water is shown for a constant FIO2
of 21%. At increasing depth, the inspired PO2
eventually exceeds the pulmonary toxic limit (≅14 m in depth) and the central
nervous system toxic limit (≅70 m in depth). The threshold for high-pressure
nervous syndrome and pressure reversal of anesthesia (observed in non-narcotic atmospheres)
is around 150 to 200 m in depth. The shaded red bars
represent the depth or altitude ranges over which risk progresses from low (light
shading) to high (dark shading). AMS,
acute mountain sickness; HACE, high-altitude cerebral edema; HAPE, high-altitude
pulmonary edema. Figure 70-3
Blood O2
content versus PO2
.
Virtually complete saturation of hemoglobin with O2
occurs at a PO2
of 100 mm Hg. Further increases in PO2
do not alter the quantity of O2
bound to hemoglobin. However, there is
a linear increase in total blood O2
content with PO2
because of increasing quantities of O2
dissolved in plasma.
Figure 70-3
Blood O2
content versus PO2
.
Virtually complete saturation of hemoglobin with O2
occurs at a PO2
of 100 mm Hg. Further increases in PO2
do not alter the quantity of O2
bound to hemoglobin. However, there is
a linear increase in total blood O2
content with PO2
because of increasing quantities of O2
dissolved in plasma.