CRITICAL CARE MANAGEMENT (also
see Chapter 74
and Chapter
75
)
Mechanical Ventilation after Trauma
Research has delineated many of the etiologies of acute lung injury
and ARDS. Classic risk factors associated with trauma are listed in Table
63-18
.[231]
[232]
[233]
Recent studies have further clarified the
role that mechanical ventilation plays in these pathologies. It is now apparent
that inappropriate ventilatory strategies can lead to lung injury.[234]
A patient who has suffered a traumatic injury is at increased risk for several reasons.
Physiologic Changes
After injury, the patient is immobilized in the supine position
on a long backboard and may remain in this position for several hours during prehospital
transport and initial diagnostic workup. Dependent atelectasis is seen early in
trauma patients as a result of these positioning restrictions. Sedation and analgesia
further exacerbate atelectasis because of cephalad movement of the diaphragm into
the thoracic cavity and compression of dorsal/dependent lung regions. General anesthesia
exacerbates this problem, and the addition of neuromuscular blockade worsens it further.
[235]
Obesity will compound atelectasis because
of marginal baseline functional residual capacity. In patients with rib fractures,
"splinting" as a result of pain will lead to atelectasis and subsequent hypoxemia.
Pulmonary contusions, if severe, can be life-threatening early in the hospital course,
and the disruption in pulmonary integrity may be exacerbated by the need for massive
blood and crystalloid replacement, thus adding further insult to the already "leaky"
capillaries and parenchyma.
Invasive Mechanical Ventilation
It is now recognized that mechanical ventilation can initiate
lung injury. Pathologic analyses from animal studies that induce pulmonary damage
to simulate acute lung injury or ARDS cannot be distinguished from the alveolar damage
occurring after studies that use mechanical ventilation strategies to induce injury.
[236]
High peak inspiratory pressures and low PEEP
settings can lead to inflammatory processes in the lung that disseminate throughout
the body and eventually cause MOSF. Acute lung injury results in an alteration in
lung micromechanics that leads to uneven distribution of ventilation.[237]
Uneven distribution of ventilation results in a chaotic pattern of pressure and
volume changes as the lung inflates and deflates.[238]
Alveolar instability leads to dyssynchronous recruitment followed by derecruitment
during cyclic ventilation. The heterogeneity of lung units in acute lung injury
results from multiple forces that create a range of opening and closing pressures.
The net result is nonuniform distribution of ventilation, which potentiates alveolar
stress failure and ultimately affects gas exchange. Overdistention is more likely
to occur in healthier, nondependent regions where the bulk of ventilation is delivered.
Shear forces develop in dependent lung regions as airspaces are cyclically recruited
and derecruited. The early stages of ventilator-associated lung injury develop at
commonly used airway pressures (transalveolar pressure >35 cm H2
O)
in animals
with normal lungs. The threshold for lung injury may occur at lower pressure in
injured lungs. Ideally, ventilator management should distribute pressure and volume
to dependent and nondependent regions proportionally.
PEEP should be applied to sustain recruitment of as many alveoli
as possible in order to maximize gas exchange and improve the distribution of ventilation.
Levels of PEEP required to maintain end-expiratory lung volume and limit shear forces
may be substantial (>20 cm H2
O). Although the exact level of PEEP
needed to eliminate cyclic airway closure and eliminate shear forces completely is
unknown, recent data suggest that within an acutely injured lung, a spectrum of airway
closing and opening pressures exists. Several researchers have published data showing
that ventilator management with low tidal volumes (6 to 8 mL/kg), limiting distending
pressures (transpulmonary or plateau pressure <35 mm Hg), and setting PEEP above
the lower inflection point on the pressure-volume curve may decrease mortality, reduce
ICU length of stay, and decrease ventilator days.[239]
[240]
[241]
Recommendations
for ventilator management, culled from several randomized, prospective trials, are
suggested in Table 63-19
.
These recommendations refer to both intraoperative
and ICU locations. For patients with respiratory failure who require surgery, if
the ventilator settings in the ICU exceed the capability of the OR ventilator, the
patient should be taken to the OR on the ICU ventilator and remain on the ICU ventilator
for the surgical procedure. Alternatively, the procedure can be performed at the
bedside in the ICU.
The combination of traumatic lung injury, fluid resuscitation,
and supine positioning results in alveolar damage, alveolar collapse (derecruitment),
worsening gas exchange, and declining compliance ( Fig.
63-13
). A shift of the pressure-volume curve to the right, seen with worsening
compliance, requires adjustments of the ventilator to prevent both low-end shear
and high-end overdistention. As ARDS develops, less of the lung parenchyma is recruitable,
compliance worsens, and the physician is often left with progressively increasing
peak airway pressures in an attempt to oxygenate the patient. Newer modalities,
such as airway pressure release ventilation (APRV), negate the need for paralysis
or deep sedation by allowing spontaneous breathing throughout the respiratory cycle.
Spontaneous breathing significantly improves ventilation-perfusion matching, cardiac
output, CO2
clearance, and renal blood flow.[242]
An investigation comparing two modes of ventilation studied 18
patients with acute lung injury maintained for 24 hours on volume-controlled inverse
ratio ventilation and then for 24 hours on APRV.[243]
Eleven of the subjects were multiple trauma patients with lung contusions or ARDS.
In addition to confirming previous study findings of a significant decrease in peak
inspiratory pressure (P < .01), a further decrease
in peak inspiratory pressure was seen after 24 hours (P
< .05), probably because of effective recruitment over time. The alveolar-arterial
gradient and venous admixture also improved with APRV (P
< .01). This study was the first to report a decrease in the use of sedative
agents during APRV. Patients treated by inverse ratio ventilation required deep
sedation, but those treated by APRV were comfortable with much lower
TABLE 63-19 -- Recommendations for ventilator settings in acutely injured patients
|
Tidal volumes of 6–8 mL/kg |
|
PEEP higher than the lower inflection point (in the most critically
ill, those with massive volume resuscitation, ALI, or ARDS, a minimum of 10–15
cm H2
O is recommended) |
|
Limit peak/plateau pressure to <40/35 cm H2
O (patients
with burn injuries, morbid obesity, or massive volume resuscitation may require higher
pressure to adequately distend the lung because much of the measured distending pressure
is actually needed to lift the restricted chest wall away from the thoracic cavity) |
|
Adjust the I:E ratio and respiratory rate as needed to achieve
the above recommendations |
|
Wean FIO2
to obtain
a PaO2
of 80–100 (or oxygenation
saturation of 93%–97%) |
|
Convert to pressure-controlled inverse-ratio ventilation if necessary
or transfer the patient to an ICU ventilator |
|
ALI, acute lung injury; ARDS, acute respiratory distress syndrome;
ICU, intensive care unit; I:E ratio, inspiratory-to-expiratory ratio; PEEP, positive
end-expiratory pressure. |
doses and were not given paralytics. APRV has also been studied in trauma patients
with ARDS, again with findings of decreased peak inspiratory pressure and higher
airway pressure, without derangements in gas exchange.[244]
Noninvasive Mechanical Ventilation
NIPPV may be an alternative to endotracheal intubation in certain
trauma patients (i.e., those without facial injuries, with a mental status that permits
both cooperation and the ability to protect the airway, and with a low risk of aspiration).
Either a nasal mask or a facemask may be used. NIPPV is a treatment modality used
most commonly in children and in patients with chronic respiratory diseases.
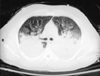 Figure 63-13
Computed tomographic chest scan of a recently injured
trauma patient showing profound consolidation of the posterior lung segments.
Figure 63-13
Computed tomographic chest scan of a recently injured
trauma patient showing profound consolidation of the posterior lung segments.
Recently, several researchers have shown improved outcomes when patients with acute
respiratory failure were managed in this manner. A prospective, randomized trial
of 64 patients with hypoxemic respiratory insufficiency demonstrated fewer serious
complications (38% versus 66%), a lower incidence of pneumonia or sinusitis (3% versus
31%), shorter periods of ventilation, and shorter stays in the ICU.[207]
Only 6% of patients initially randomized to NIPPV were eventually intubated. A
retrospective review of trauma patients with acute respiratory failure showed an
improvement in the PaO2
/FIO2
ratio, an increase in tidal volume, and a decrease in the respiratory rate with a
mean pressure support level of 12 cm H2
O and PEEP of 4.5 cm H2
O
applied by facemask. The length of time for the use of NIPPV was 6 to 144 hours.
[208]
 Figure 63-13
Computed tomographic chest scan of a recently injured
trauma patient showing profound consolidation of the posterior lung segments.
Figure 63-13
Computed tomographic chest scan of a recently injured
trauma patient showing profound consolidation of the posterior lung segments.