Cardiovascular System
The aging process is associated with primary and secondary changes
in the heart, as well as primary changes in the blood vessels and alterations in
autonomic control (see Chapter 18
).
As the heart ages, changes in morphology occur. For instance, myocyte number decreases,
left ventricular wall thickening occurs, and both conduction fiber density and sinus
node cell number decrease.[7]
Functionally, these
changes translate to decreased contractility, increased myocardial stiffness and
ventricular filling pressure, and decreased β-adrenergic sensitivity.[7]
Vascular stiffness increases with advancing age. Specifically, breakdown of elastin
and collagen leads to changes in the vascular wall matrix that result in increased
medial and intimal thickness. Morphologically, one sees an increase in the diameter
and stiffness of large elastic arteries. Functionally, these changes are readily
observed in terms of an elevated mean arterial pressure and increased pulse pressure.
[8]
Increased vascular stiffness leads to important
secondary responses in the heart.
The vascular system functions as both a cushion and a conduit
to ensure mechanically efficient and smooth delivery of blood to the periphery.
In youth, the cardiac pump and the blood vessels are optimally coupled to maximize
efficiency.[9]
With increased resistance in blood
vessels, the velocity of conduction of pulse waves down the vascular tree increases.
Increased pulse wave velocity results in earlier reflection of pulse waves from
the periphery. In younger humans, wave reflection occurs later because of slower
propagation such that reflected waves reach the heart after aortic valve closure.
In the setting of increased pulse wave velocity and early wave reflection, reflected
pulse waves reach the heart during the latter phases of ejection, thereby imposing
an
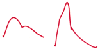 Figure 62-1
Pressure wave pattern as seen in young human adults and
in experimental animals (left) contrasted to pressure wave pattern as seen in mature
human adults (right). (Reprinted with permission from O'Rourke MF, Kelly
RP, Avolio AP [eds]: The Arterial Pulse. Philadelphia, Lea & Febiger, 1992.)
Figure 62-1
Pressure wave pattern as seen in young human adults and
in experimental animals (left) contrasted to pressure wave pattern as seen in mature
human adults (right). (Reprinted with permission from O'Rourke MF, Kelly
RP, Avolio AP [eds]: The Arterial Pulse. Philadelphia, Lea & Febiger, 1992.)
increased cardiac load.[7]
This effect can be observed
by the late systolic peaking evident on the arterial pressure waveform ( Fig.
62-1
).[10]
This increase in left ventricular
afterload leads to left ventricular wall thickening and hypertrophy. Left ventricular
wall thickening and increased afterload combine to cause a compensatory prolongation
in myocardial contraction. Such prolongation occurs at the expense of decreased
early diastolic filling time. Under these conditions, the contribution of atrial
contraction to late ventricular filling becomes more important and explains why cardiac
rhythm other than sinus is often poorly tolerated in the elderly. In elderly men,
an elevated end-diastolic volume preserves stroke volume. Aging women have a mild
decrease in cardiac output. The changes in cardiovascular physiology that occur
in healthy persons with aging are summarized in Table
62-2
.
The two most important changes in the autonomic system with aging
are a decrease in response to β-receptor stimulation and an increase in sympathetic
nervous system activity. Decreased β-receptor responsiveness is secondary to
both decreased receptor affinity and alterations in signal transduction.[11]
Decreased β-receptor responsiveness assumes functional importance when increased
flow demands are placed on the heart. Normally, β-receptor-mediated mechanisms
act to increase the heart rate, venous return, and systolic arterial pressure while
preserving preload reserve. In contrast, the attenuated β-receptor response
in the elderly during exercise/stress is associated with a decreased maximal heart
rate and decreased peak ejection fraction. Such decreases cause the increased peripheral
flow demand to be met primarily by preload reserve, thereby making the heart more
susceptible to cardiac failure.[7]
It is well known
that sympathetic nervous system activity increases with aging. Although changes
in β-receptor responsiveness are well defined, it is controversial whether the
aging process alters the α-receptor response. Increased resting sympathetic
nervous system activity may contribute to increases in systemic vascular resistance
along with mechanical stiffening of the peripheral vasculature.[7]
Clinically, these autonomic changes lead to a greater likelihood of intraoperative
hemodynamic lability and a decreased ability to meet the metabolic demands of surgery.
Although the age-related changes in cardiovascular physiology
are generally well tolerated, several pathophysiologic states deserve mention. For
one, impairment of diastolic relaxation leads to diastolic dysfunction in the aging
heart. In its severest form, diastolic dysfunction may be manifested as diastolic
heart failure. Predisposing disease states for this condition include hypertension
with left ventricular hypertrophy, ischemic heart disease, hypertrophic cardiomyopathy,
and valvular heart disease. The problem occurs when decreased left ventricular compliance
during diastole results in greatly increased left ventricular diastolic pressure.
If this pressure is conducted retrogradely to the pulmonary circulation, pulmonary
venous congestion and pulmonary edema result. Diastolic dysfunction/failure is often
related to systemic blood pressure and does not necessarily imply volume overload.
The diagnosis can be difficult to make because the clinical picture appears identical
to left ventricular systolic failure. Making the correct diagnosis is important
in that interventions commonly used in systolic failure such as diuretics and inotropes
may exacerbate diastolic dysfunction. Echocardiography is the diagnostic modality
of choice.[12]
Classically, echocardiography will
demonstrate preserved or hyperdynamic left ventricular systolic function and characteristic
changes in flow velocity at the mitral valve.
Aortic valve sclerosis and mitral annular calcification are common
echocardiographic findings in the elderly. They represent non-flow-limiting calcifications
around the aortic and mitral valves, respectively. Otto and coworkers demonstrated
that aortic valve sclerosis is common in the elderly and is associated with a 50%
increase in the risk of cardiovascular death.[13]
Hence, it has been suggested that aortic valve sclerosis may represent a marker
for coronary artery disease. A similar association has been postulated for mitral
annular calcification.[14]
 Figure 62-1
Pressure wave pattern as seen in young human adults and
in experimental animals (left) contrasted to pressure wave pattern as seen in mature
human adults (right). (Reprinted with permission from O'Rourke MF, Kelly
RP, Avolio AP [eds]: The Arterial Pulse. Philadelphia, Lea & Febiger, 1992.)
Figure 62-1
Pressure wave pattern as seen in young human adults and
in experimental animals (left) contrasted to pressure wave pattern as seen in mature
human adults (right). (Reprinted with permission from O'Rourke MF, Kelly
RP, Avolio AP [eds]: The Arterial Pulse. Philadelphia, Lea & Febiger, 1992.)