|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Preeclampsia complicates up to 8% of pregnancies.[233] It is the most common condition seen by obstetric anesthesiologists in which an otherwise healthy parturient can become critically ill. The classic triad of preeclampsia includes hypertension, proteinuria, and edema. Certain conditions predispose a woman to preeclampsia. In order of risk ratio, these conditions include homozygous angiotensin T-235, chronic renal disease, antiphospholipid syndrome, chronic hypertension, a family history of preeclampsia, multiple gestation, nulliparity, maternal age older than 40 years, diabetes, and African American race.[234] Preeclampsia has been defined as hypertension occurring after 20 weeks' gestation or in the early postpartum period and returning to normal within 3 months after delivery or onset after 20 weeks' gestation and at least one of the following:[235]
Preeclampsia may be classified as mild or severe according to the severity of symptoms and signs as illustrated in Table 58-9 . It is misleading to assume a smooth progression from mild disease to severe preeclampsia to eclampsia because 25% to 40% will have normal blood pressure at the time of their first eclamptic seizure.[236]
In the most recent U.K. report "Why Mothers Die 1997–1999," hypertensive disease is the second leading cause of direct deaths. It states "intra-cranial hemorrhage is the largest single cause of death in preeclampsia/eclampsia, reflecting a failure of effective anti-hypertensive therapy."[237]
Despite intensive research, the precise cause of preeclampsia
remains unknown, thus making it "the disease of theories." Preeclampsia may be associated
with abnormal placentation and failure of the normal invasion of trophoblast cells,
thereby leading to maladaptation of maternal spiral arteries.[238]
This aberration can result in abnormal villus development and potentially cause
placental insufficiency. It has recently been proposed that the secondary pathology
in preeclampsia is also linked to endothelial dysfunction and excessive activation
of coagulation. This abnormality is accompanied by high circulating levels of fibronectin
and endothelin, increased
|
|
Mild | Severe |
|---|---|---|
| Systolic arterial pressure | <160 mm Hg | ≥160 mm Hg |
| Diastolic arterial pressure | <110 mm Hg | ≥110 mm Hg |
| Urinary protein | <5 g/24 hr | ≥5 g/24 hr |
|
|
Dipstick + or 2 + | Dipstick 3+ or 4+ |
| Urine output | >500 mL/24 hr | ≤500 mL/24 hr |
| Headache | No | Yes |
| Visual disturbances | No | Yes |
| Epigastric pain | No | Yes |
| Right upper quadrant abdominal pain | No | Yes |
| Pulmonary edema | No | Yes |
| Cyanosis | No | Yes |
| HELLP | No | Yes |
| Platelet count | >100,000/mm3 | <100,000/mm3 |
| HELLP, hemolysis, elevated liver enzymes, and low platelet count. | ||
| From Birnbach DJ, Gatt SP, Datta S (eds): Textbook of Obstetric Anesthesia. New York, Churchill Livingstone, 2000, p 543. | ||
Several studies have focused on the possible genetic basis of preeclampsia. It is thought that a single preeclampsia gene is unlikely, but that there are probably many modifier genes in conjunction with environmental factors. [241]
The upper airway may become edematous in a preeclamptic woman and result in the potential for airway compromise
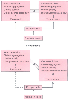 Figure 58-13
Comparison of the normal equilibrium and actions of thromboxane
and prostacyclin in normal pregnancy with the dysequilibrium associated with preeclampsia.
(Redrawn from Walsh SW: Preeclampsia: An imbalance in placental prostacyclin
and thromboxane production. Am J Obstet Gynecol 152:335, 1985.)
Figure 58-13
Comparison of the normal equilibrium and actions of thromboxane
and prostacyclin in normal pregnancy with the dysequilibrium associated with preeclampsia.
(Redrawn from Walsh SW: Preeclampsia: An imbalance in placental prostacyclin
and thromboxane production. Am J Obstet Gynecol 152:335, 1985.)
The cardiovascular effects of preeclampsia may vary. Cotton and colleagues[243] described three subsets of cardiovascular changes in women with severe untreated preeclampsia:
In preeclampsia, increased uric acid results from decreased renal excretion and reflects tissue ischemia and oxidative stress. Acute renal failure is rare, but it may occur, especially with HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets) and placental abruption. Hepatic impairment may also occur, but it is usually associated with HELLP syndrome. Liver rupture is a known,
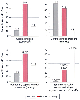 Figure 58-14
Hemodynamic subsets in 45 untreated parturients with
severe preeclampsia. (Redrawn from Cotton DB, Lee W, Huhta JC, et al: Hemodynamic
profile of severe pregnancy induced hypertension. Am J Obstet Gynecol 158:523–529,
1988.)
Figure 58-14
Hemodynamic subsets in 45 untreated parturients with
severe preeclampsia. (Redrawn from Cotton DB, Lee W, Huhta JC, et al: Hemodynamic
profile of severe pregnancy induced hypertension. Am J Obstet Gynecol 158:523–529,
1988.)
As stated, this condition consists of hemolysis, elevated liver enzymes, and low platelets. Its severity ranges from a mild self-limiting condition to a fulminant process leading to multiorgan failure. Considerable debate, however, has centered around the definition, incidence, etiology, and management of this syndrome. Patients with HELLP syndrome may have various signs and symptoms, none of which are diagnostic and all of which may be found in patients with severe preeclampsia/eclampsia without HELLP syndrome.[244] Sibai, noting the lack of consensus regarding the diagnostic features of HELLP syndrome, recommended the following criteria: hemolysis (defined by an abnormal peripheral blood smear and an increased bilirubin level), increased liver enzymes (defined as an aspartate aminotransferase level of at least 70 U/L and LDH greater than 600 U/L), and a low platelet count (less than 100,000/mm3 ).[245]
Assuming no other coagulopathy, hemostasis is typically not problematic unless the platelet count decreases below 40,000/mm3 .[246] The rate of fall in the platelet count is also of clinical significance, and regional anesthesia may be contraindicated if the platelet count has dramatically fallen over a short period. The platelet count usually returns to normal within 72 hours of delivery, but thrombocytopenia may persist for longer periods.
Once diagnosed, the goal of therapy in a preeclamptic patient is prevention and reduction of further complications by taking into account both maternal and fetal factors. Although the only definitive cure is delivery, management of maternal hemodynamics and prevention of the development of eclampsia are key to a favorable outcome for the mother and baby. There is now international consensus that magnesium is the treatment of choice for preeclampsia to prevent eclampsia, but the mechanism underlying its salutary effect remains debatable. Although magnesium is a direct smooth muscle relaxant at relatively high concentrations, it does not significantly reduce systemic blood pressure at the serum concentrations that are efficacious in treating preeclampsia.[247]
The mainstay of therapy in preeclampsia is control of hypertension, prevention of seizures, and delivery of the fetus. Hydralazine and labetalol are commonly used as antihypertensives ( Table 58-10 ). Other agents include nitroglycerin, nifedipine, and esmolol. Sodium nitroprusside may also be used, but only in the short term because of a risk of cyanide toxicity in the fetus.
Magnesium sulfate is the agent of choice for seizure control and prevention of recurrent eclamptic seizures.[248] [249] The recently published Magpie Trial demonstrated the efficacy of MgSO4 in seizure prophylaxis. It was shown to reduce seizures by more than 50% and without any serious maternal morbidity.[250] A beneficial secondary effect in some patients may be vasodilatation and an increase in cardiac output by reducing SVR. Initial dosing is 4 g MgSO4
| Drug | Hydralazine | Labetalol |
|---|---|---|
| Mode of action | Vasodilator | α- and β-blocker (1:3) |
| Speed of onset | Gradual | Quick |
| Dose | 5–10 mg IV slowly | 10–20 mg IV slowly |
| Interval | Repeat after 20 min | Titrate to effect |
| Infusion rate | 2 mg/hr up to 20 mg/hr | 20 mg/hr to maximum of 160 mg/hr |
| Effect on heart rate | Compensatory tachycardia | No effect |
Anesthetic management of a preeclamptic patient includes a detailed preanesthetic assessment that focuses on the severity of the condition, associated features and systemic involvement, evaluation of the airway, fluid status, and blood pressure control. Investigations should include a complete blood count, renal profile, and liver function tests. Routine coagulation screening is not recommended by all authors,[251] but if coagulopathy is suspected clinically, coagulation studies should be performed. However, before considering neuraxial analgesia or anesthesia, a recent platelet count must be evaluated. DIC may require
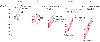 Figure 58-15
Initial central venous pressure (C.V.P.) readings (three
or more recordings of maternal diastolic pressure) and intravascular volume replacement
required to attain the range of 6 to 8 cm H2
O in five groups of women
with preeclampsia classified according to the severity of the disease (by diastolic
blood pressure). LR, lactated Ringer's solution. (Redrawn from Joyce TH
III, Debnath KS, Baker EA: Preeclampsia: Relationship of CVP and epidural analgesia.
Anesthesiology 51[Suppl]:294, 1979.)
Figure 58-15
Initial central venous pressure (C.V.P.) readings (three
or more recordings of maternal diastolic pressure) and intravascular volume replacement
required to attain the range of 6 to 8 cm H2
O in five groups of women
with preeclampsia classified according to the severity of the disease (by diastolic
blood pressure). LR, lactated Ringer's solution. (Redrawn from Joyce TH
III, Debnath KS, Baker EA: Preeclampsia: Relationship of CVP and epidural analgesia.
Anesthesiology 51[Suppl]:294, 1979.)
Although preeclampsia is accompanied by exaggerated retention of water and sodium, hypovolemia may be present because of a shift of fluids and proteins into the extravascular compartment. An inverse relationship between intravascular volume and the severity of hypertension has been demonstrated, and patients with very elevated diastolic pressure can be expected to have negative central venous pressure readings ( Fig. 58-15 ).
Careful volume expansion may result in improved maternal tissue perfusion. As illustrated in Table 58-11 , fluid administration is accompanied by a significant increase in pulmonary capillary wedge pressure and cardiac index and a decrease in SVR and maternal heart rate. In the presence of severe hypertension, central venous pressure may not be an acceptable measurement of right-sided preload.
Debate has been ongoing regarding the use of pulmonary artery catheters, and information about their use in the obstetric population is lacking. In 1997, The Pulmonary Artery Catheter Consensus recommended that the pulmonary artery catheter not be routinely used in the management of preeclampsia because of the lack of evidence supporting its use.[252] However, a retrospective study examining the safety and efficacy of pulmonary artery catheters in the management of severe preeclampsia and eclampsia reported a subjective benefit with their use in 93% of cases with a complication rate of 4%. The most common indications for insertion included renal failure (53%), pulmonary edema (30%), and eclampsia (17%).[253]
Epidural labor analgesia in a preeclamptic patient offers the advantage of a gradual onset of sympathetic blockade; it gives cardiovascular stability and avoids neonatal depression. The reduction in vasospasm and hypertension achieved potentially results in improved uteroplacental blood flow. Regional techniques also reduce the risk of airway complications and avoid the hemodynamic alterations associated with intubation. Ramanathan and coauthors reported the use of a CSE technique using 1.25 mg bupivacaine and 25 µg fentanyl in 39 severe preeclamptic parturients for labor analgesia. All patients had adequate analgesia with a maximum decrease in mean arterial
|
|
Preeclamptic Patients (n = 10) |
|
||||
|---|---|---|---|---|---|---|
| Variable | Initial | After Volume Expansion | P * | After Vasodilation | P † | Control Subjects (n = 4) |
| Diastolic blood pressure (mm Hg) | 106 (100–120) | 102 (90–120) | NS | 85 (75–100) | <.01 | 77 (70–90) |
| Mean arterial pressure (mm Hg) | 121 (113–136) | 116 (103–136) | <.02 | 102 (97–116) | <.01 | 95 (93–106) |
| Heart rate (beats/min) | 100 (90–130) | 81 (60–110) | <.02 | 82 (70–100) | NS | 84 (70–90) |
| Pulmonary capillary wedge pressure (mm Hg) | 3.3 (1–5) | 8 (7–10) | <.01 | 8 (7–9) | NS | 9 (6–12) |
| Systemic vascular resistance (dyne/sec/cm5 ) | 1943 (1480–2580) | 1284 (1073–1600) | <.01 | 947 (782–1028) | <.01 | 886 (805–1021) |
| Cardiac index (L/min/m2 ) | 2.75 (1.97–3.33) | 3.77 (3.26–4.05) | <.01 | 4.40 (3.94–5.00) | <.01 | 4.53 (3.96–4.97) |
| NS, not significant, Wilcoxon signed-rank test (two-tailed). | ||||||
| From Groenendijk R, Trimbos MJ, Wallenberg HCS: Hemodynamic measurements in pre-eclampsia: Preliminary observations. Am J Obstet Gynecol 150:232, 1984. | ||||||
Although some authors have suggested that neuraxial anesthesia not be routinely performed in women with severe preeclampsia,[255] data suggest otherwise. A report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy from the National Heart, Lung, and Blood Institute has stated the following:
Neuraxial (epidural, spinal and combined spinal-epidural) techniques offer many advantages for labor analgesia and can be safely administered to the parturient with preeclampsia. Dilute epidural infusions of local anesthetic plus opioid produce adequate sensory block without motor block or clinically significant sympathectomy. When neuraxial techniques are used for cesarean delivery, however, there is a possibility of extensive sympatholysis with profound hypotension which may lead to decreased cardiac output and further diminished uteroplacental perfusion. This may be more likely with single shot spinal anesthesia, which although considered acceptable by some experts, is still considered by others to be relatively contraindicated for women with severe preeclampsia. A recent analysis, however, suggests that spinal anesthesia can be safely used in the patient with severe preeclampsia undergoing cesarean delivery because the magnitude of declines in maternal blood pressure after spinal and epidural anesthesia appear to be similar.[256] Hypotension can usually be avoided by meticulous attention to anesthetic technique and careful volume expansion. In one unblinded study of 80 women with severe preeclampsia randomly assigned to receive epidural, CSE, or general anesthesia, all three regimens appeared equally safe.[257] With general anesthesia, significant hypertension may occur at the time of laryngoscopy, at tracheal intubation, and again during emergence and extubation period. These responses can usually be blocked by appropriate pre-treatment with hydralazine, nitroglycerin, or labetalol.
Airway edema may be seen in the patient with preeclampsia and may increase the risks of a difficult airway situation, leading to failed intubation and ventilation. Because general anesthesia poses considerably greater risk to parturients than regional anesthesia, the risk of a failed intubation must be weighed against the risk of transient hypotension when deciding between general and regional anesthesia for cesarean delivery for the patients with severe preeclampsia-eclampsia. Although neuraxial techniques have become the preferred method to provide labor analgesia or anesthesia for cesarean delivery among women with severe preeclampsia-eclampsia, they are relatively contraindicated in the presence of coagulopathy. Early consultation with an anesthesiologist is suggested for parturients with severe preeclampsia.[258]
If general anesthesia becomes necessary in a preeclamptic patient receiving MgSO4 therapy, the activity of succinylcholine will be potentiated; there is also enhanced sensitivity to non-depolarizing muscle relaxants. Magnesium offers another advantage in that it blunts the response to vasoconstrictors and inhibits catecholamine release after sympathetic stimulation.
|
|
|
|
|
|
|
|
|
|
|
|
|