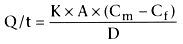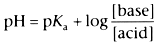PLACENTAL TRANSFER OF ANESTHETIC DRUGS
Many medications administered to a pregnant woman cross the placenta,
and these medications may have far-reaching
effects on the fetus. Commonly used medications have both direct pharmacologic actions
and indirect effects on the in utero environment. After administration of a medication
to the mother, a certain amount will cross the placenta and enter the fetal circulation.
Drugs cross the placenta by three main processes: simple diffusion, active transport,
or pinocytosis. The extent of drug transfer is dependent on numerous factors, including
molecular weight, protein binding, degree of lipid solubility, maternal drug concentration,
and maternal and fetal pH. The Fick principle governs the rate of transfer of a
drug across a membrane:
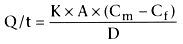
where Q/t = rate of diffusion, K = diffusion coefficient, A = surface area of membrane
available for exchange, Cm
- Cf
= concentration gradient between
the maternal and fetal circulations, and D = thickness of the membrane.
Larger molecules are less likely to cross the placenta, but those
with weights under 500 daltons will cross easily. Most drugs administered to a parturient
in labor have low molecular weights; therefore, they transfer easily to the fetus.
Drugs with high lipid solubility will also readily cross the placenta. Highly ionized
substances with poor lipid solubility (such as non-depolarizing muscle relaxants)
have limited transfer.
Once a drug crosses the placenta, fetal pH and protein binding
affect drug disposition.[44]
The degree of ionization
greatly influences drug transfer because only non-ionized portions of the drug can
cross the placenta.
The degree of ionization of a drug is determined by the Henderson-Hasselbalch
equation:
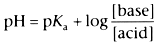
where pKa
is the negative logarithm of
the acid dissociation constant. The pKa
of a drug is the pH at which it is 50% ionized and 50% un-ionized. Most local anesthetic
pKa
values range from 7.7 to 9.1, which
is near physiologic pH values. Changes in maternal and fetal blood pH may alter
the extent of ionization of a drug and its placental transfer. A phenomenon known
as "ion trapping" may occur in a fetus with acidosis because lower fetal pH favors
ionization of basic local anesthetics (such as lidocaine) and may explain the accumulation
of drugs in a compromised fetus.[45]
Because of its unique characteristics, the fetal circulation can
have a major impact on drug distribution within the fetus. After drugs cross the
placenta, they enter the fetal circulation through the umbilical vein. The liver
is the first fetal organ perfused by umbilical venous blood, and substantial hepatic
uptake has been described for many drugs. However, approximately 40% of the umbilical
venous blood bypasses the liver.[46]
Hepatic drug
uptake in the fetus may protect the fetus from the deleterious effects of certain
drugs on the central nervous system.[47]
Dilution
of umbilical venous blood across the foramen ovale and ductus arteriosus may also
modify fetal drug distribution.