Increase in the Partial Pressure of Arterial Carbon
Dioxide
During uneventful CO2
pneumoperitoneum, the increase
in partial pressure of arterial carbon dioxide (PaCO2
)
progressively increases to reach a plateau 15 to 30 minutes after the beginning of
CO2
insufflation in patients under controlled mechanical ventilation during
gynecologic laparoscopy in the Trendelenburg position[19]
or laparoscopic cholecystectomy in head-up position[20]
[21]
( Fig.
57-2
). Any significant increase in PaCO2
after this period requires a search for a cause independent of or related to CO2
insufflation, such as CO2
subcutaneous emphysema. The increase in PaCO2
depends on the IAP.[22]
During laparoscopy with
local anesthesia, PaCO2
remains unchanged,
but minute ventilation significantly increases.[23]
However, during general anesthesia with spontaneous breathing, the compensatory
hyperventilation is insufficient to avoid hypercapnia because of anesthetic-induced
ventilatory depression and increased work of breathing from the decreased thoracopulmonary
compliance. Because it takes 15 to 30 minutes for PaCO2
to plateau, anesthetic techniques using spontaneous breathing should be limited to
short procedures at low IAPs.[24]
[25]
Capnography and pulse oximetry provide reliable monitoring of
PaCO2
and arterial oxygen saturation in
healthy patients and in the absence of acute intraoperative disturbances (see Fig.
57-2
).[17]
[20]
[21]
Although mean gradients (Δa-ETCO2
)
between PaCO2
and the end-tidal carbon
dioxide tension (PETCO2
) do not change
significantly during peritoneal insufflation of CO2
, individual patient
data regularly show variations of this difference during pneumoperitoneum.[26]
[27]
PaCO2
and Δa-ETCO2
increase more in ASA
class II and III patients than ASA class I patients ( Fig.
57-3
).[28]
[29]
These findings have been documented in patients with chronic obstructive disease
(COPD)[30]
and in children with cyanotic congenital
heart disease.[31]
These data therefore highlight
the lack of correlation between PaCO2
and PETCO2
in sick patients, particularly
those with impaired CO2
excretion capacity, and in otherwise healthy patients
with acute cardiopulmonary disturbances. Consequently, arterial blood sampling is
recommended when hypercapnia is clinically suspected, even in the absence of abnormal
PETCO2
. Postoperative intra-abdominal
CO2
retention results in increased respiratory rate and PETCO2
of patients breathing spontaneously after laparoscopic cholecystectomy as compared
with open cholecystectomy.[32]
During CO2
pneumoperitoneum, the increase of PaCO2
may be multifactorial: absorption of CO2
from the peritoneal cavity;
impairment of pulmonary ventilation and perfusion by mechanical factors such as abdominal
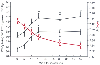 Figure 57-2
Ventilatory changes (pH, PaCO2
,
and PETCO2
) during carbon dioxide pneumoperitoneum
for laparoscopic cholecystectomy. For 13 American Society of Anesthesiologists (ASA)
class I and II patients, minute ventilation was kept constant at 100 mL/kg/min with
a respiratory rate of 12 per minute during the study. Intra-abdominal pressure was
14 mm Hg. Data are given as the mean ± SEM.*, P
< .05 compared with time 0.
Figure 57-2
Ventilatory changes (pH, PaCO2
,
and PETCO2
) during carbon dioxide pneumoperitoneum
for laparoscopic cholecystectomy. For 13 American Society of Anesthesiologists (ASA)
class I and II patients, minute ventilation was kept constant at 100 mL/kg/min with
a respiratory rate of 12 per minute during the study. Intra-abdominal pressure was
14 mm Hg. Data are given as the mean ± SEM.*, P
< .05 compared with time 0.
distention, patient position, and volume-controlled mechanical ventilation; and depression
of ventilation by premedicant and anesthetic agents in the case of spontaneous breathing
( Table 57-1
). The observation
of an increase in PaCO2
when CO2
,
but not nitrous oxide (N2
O) or helium, was used as the insufflating gas
suggests that the main mechanism of the increased PaCO2
during CO2
pneumoperitoneum is absorption of CO2
rather than
the mechanical ventilatory repercussions of increased IAP.[33]
[34]
[35]
Accordingly,
direct measurement of CO2
elimination (V̇CO2
)
using a metabolic monitor combined with investigation of gas exchange showed a 20%
to 30% increase of V̇CO2
without significant
changes in physiologic dead space in healthy patients undergoing pelvic laparoscopy
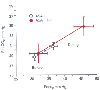 Figure 57-3
Ventilatory changes as a function of patient physical
status. The partial pressure of arterial carbon dioxide (PaCO2
)
and end-tidal carbon dioxide tension (PETCO2
)
were measured before and during carbon dioxide insufflation. Patients were grouped
according to ASA classification: group 1 (open circles),
ASA I (n = 20); group 2 (red circles), ASA II–III
(n = 10). (Data from Wittgen CM, Andrus CH, Fitzgerald SD, et al: Analysis
of the hemodynamic and ventilatory effects of laparoscopic cholecystectomy. Arch
Surg 126:997, 1991.)
Figure 57-3
Ventilatory changes as a function of patient physical
status. The partial pressure of arterial carbon dioxide (PaCO2
)
and end-tidal carbon dioxide tension (PETCO2
)
were measured before and during carbon dioxide insufflation. Patients were grouped
according to ASA classification: group 1 (open circles),
ASA I (n = 20); group 2 (red circles), ASA II–III
(n = 10). (Data from Wittgen CM, Andrus CH, Fitzgerald SD, et al: Analysis
of the hemodynamic and ventilatory effects of laparoscopic cholecystectomy. Arch
Surg 126:997, 1991.)
(IAP of 12 to 14 mm Hg) in the head-down position[16]
[19]
[36]
or laparoscopic
cholecystectomy in the head-up position.[19]
[37]
The time courses of the increase in V̇CO2
and PaCO2
are superposable. The absorption
of a gas from the peritoneal cavity depends on its diffusibility, the absorption
area, and the perfusion of the walls of that cavity. Because CO2
diffusibility
is high, absorption of large quantities of CO2
into the blood and the
subsequent marked increases in PaCO2
would
be expected to occur. The limited rise of PaCO2
actually observed can be explained by the capacity of the body to store CO2
[38]
and by impaired local perfusion due to increased
IAP.[22]
During desufflation, CO2
accumulated
in collapsed peritoneal capillary vessels reaches the systemic circulation, leading
to a transient increase in PaCO2
and V̇CO2
.
[6]
Respiratory changes during the laparoscopic procedure may contribute
to increasing CO2
tension. Mismatching of ventilation and pulmonary perfusion
can result from
TABLE 57-1 -- Causes of increased PaCO2
during
laparoscopy
|
1. Absorption of carbon dioxide (CO2
) from the peritoneal
cavity |
2. V̇A/ mismatch:
increased physiologic dead space mismatch:
increased physiologic dead space |
|
Abdominal distention |
|
Position of the patient (e.g.,
steep tilt) |
|
Controlled mechanical ventilation |
|
Reduced cardiac output |
|
These mechanisms are accentuated
in sick patients (e.g., obese, American Society of Anesthesiologists class II or
III) |
|
3. Increased metabolism (e.g., insufficient plane of anesthesia) |
|
4. Depression of ventilation by anesthetics (e.g., spontaneous
breathing) |
|
5. Accidental events |
|
CO2
emphysema
(i.e., subcutaneous or body cavities) |
|
Capnothorax |
|
CO2
embolism |
|
(Selective bronchial intubation) |
the position of the patient and the increased airway pressures associated with abdominal
distention.[23]
[39]
Lister and colleagues[22]
investigated the relationship
between V̇CO2
and intraperitoneal
CO2
insufflation pressure in pigs. For an IAP up to 10 mm Hg, increased
V̇CO2
accounts for the increased PaCO2
.
At higher IAP, the continued rise of PaCO2
without a corresponding increase in V̇CO2
results from an enlargement of respiratory dead space, as reflected by a widening
of the Δa-ETCO2
gradient.[22]
If controlled ventilation is not adjusted in response to the increased dead space,
alveolar ventilation will decrease, and PaCO2
will increase. In healthy patients, absorption of CO2
from the abdominal
cavity represents the main (or the only) mechanism responsible for increased PaCO2
,
[40]
but in patients with cardiorespiratory problems,
ventilatory changes also contribute significantly to increasing PaCO2
.
[28]
PaO2
values and intrapulmonary shunting do not change significantly during laparoscopy.
[20]
[28]
[40]
Although increased PaCO2
may be well tolerated by young, otherwise healthy patients, the extent to which hypercapnia
is acceptable has not been determined and probably varies according to the patient's
physical status. It is wise to maintain PaCO2
within physiologic ranges by adjusting controlled mechanical ventilation. Except
in special circumstances, such as CO2
subcutaneous emphysema, correction
of increased PaCO2
can be easily achieved
by a 10% to 25% increase in alveolar ventilation.
 Figure 57-2
Ventilatory changes (pH, PaCO2
,
and PETCO2
) during carbon dioxide pneumoperitoneum
for laparoscopic cholecystectomy. For 13 American Society of Anesthesiologists (ASA)
class I and II patients, minute ventilation was kept constant at 100 mL/kg/min with
a respiratory rate of 12 per minute during the study. Intra-abdominal pressure was
14 mm Hg. Data are given as the mean ± SEM.*, P
< .05 compared with time 0.
Figure 57-2
Ventilatory changes (pH, PaCO2
,
and PETCO2
) during carbon dioxide pneumoperitoneum
for laparoscopic cholecystectomy. For 13 American Society of Anesthesiologists (ASA)
class I and II patients, minute ventilation was kept constant at 100 mL/kg/min with
a respiratory rate of 12 per minute during the study. Intra-abdominal pressure was
14 mm Hg. Data are given as the mean ± SEM.*, P
< .05 compared with time 0. Figure 57-3
Ventilatory changes as a function of patient physical
status. The partial pressure of arterial carbon dioxide (PaCO2
)
and end-tidal carbon dioxide tension (PETCO2
)
were measured before and during carbon dioxide insufflation. Patients were grouped
according to ASA classification: group 1 (open circles),
ASA I (n = 20); group 2 (red circles), ASA II–III
(n = 10). (Data from Wittgen CM, Andrus CH, Fitzgerald SD, et al: Analysis
of the hemodynamic and ventilatory effects of laparoscopic cholecystectomy. Arch
Surg 126:997, 1991.)
Figure 57-3
Ventilatory changes as a function of patient physical
status. The partial pressure of arterial carbon dioxide (PaCO2
)
and end-tidal carbon dioxide tension (PETCO2
)
were measured before and during carbon dioxide insufflation. Patients were grouped
according to ASA classification: group 1 (open circles),
ASA I (n = 20); group 2 (red circles), ASA II–III
(n = 10). (Data from Wittgen CM, Andrus CH, Fitzgerald SD, et al: Analysis
of the hemodynamic and ventilatory effects of laparoscopic cholecystectomy. Arch
Surg 126:997, 1991.)