PERIOPERATIVE MANAGEMENT OF PATIENTS WITH ASYMPTOMATIC
OR CHRONIC LIVER DYSFUNCTION
The goal of perioperative management of patients with asymptomatic
elevations in aminotransaminase, alkaline phosphatase, or bilirubin levels or those
with preexisting chronic liver disease is prevention of acute liver failure or further
hepatic deterioration. Acute liver failure, defined by encephalopathy in the setting
of acute liver injury (e.g., viral hepatitis, toxin ingestion, idiosyncratic drug
reaction, sepsis, or shock), is further categorized into hyperacute, acute, or subacute
forms based on the time interval between the onset of jaundice and encephalopathy
(0 to 7 days, 8 to 28 days, >28 days, respectively).[192]
Patients with the hyperacute form of acute liver failure tend to have a more favorable
survival rate (35%), whereas those with the acute and subacute form have poorer prognoses,
with mortality rates of 93% and 86%, respectively.[192]
It is therefore imperative to identify perioperative risks and direct therapy to
minimize hepatotoxicity and maximize hepatic oxygen delivery. For example, drugs
such as acetaminophen or combined drugs such as acetaminophen/hydrocodone probably
should not be administered to patients with asymptomatic elevation of liver enzyme
test results or chronic liver dysfunction. In patients with cholelithiasis and biliary
tract obstruction, it may be prudent to limit the use of opiates preoperatively because
they have been reported to cause spasm of the choledochoduodenal sphincter (sphincter
of Oddi) and a subsequent increase in intrabiliary tract pressure.[193]
[194]
These effects appear to be more pronounced
for fentanyl, morphine, and meperidine and less severe for opiate agonistantagonists.
[193]
[194]
Intraoperatively,
opiates may be used with the recognition that the possible development of sphincter
of Oddi spasm can be treated directly with naloxone or by relaxing smooth muscle
tone with either glucagon or nitroglycerin[194]
[195]
(which induces release of nitric oxide).[196]
[197]
Remifentanil appears to have a similar effect as other opiates
on the sphincter of Oddi. Fragen and colleagues[198]
studied remifentanil given to six healthy adult
TABLE 55-4 -- Drug-induced hepatotoxicity
|
Agent |
Hepatocellular Cytotoxicity |
Steatosis |
Cholestasis |
|
Acetaminophen[134]
|
✓ |
|
|
|
Alcohol[135]
|
|
✓ |
|
|
Allopurinol[136]
|
✓ |
|
|
|
Amiodarone[137]
|
|
✓ |
|
|
Amoxicillin/clavulanic acid[138]
|
|
|
✓ |
|
Aspirin[139]
|
✓ |
✓ |
|
|
Azathioprine[138]
|
✓ |
|
✓ |
|
Bleomycin[140]
|
|
✓ |
|
|
Bosentan[141]
|
✓ |
|
|
|
Calcium channel blockers[138]
|
✓ |
|
|
|
Captopril[142]
|
|
|
✓ |
|
Carbamazepine[138]
|
✓ |
|
✓ |
|
Chlorpromazine[143]
|
|
|
✓ |
|
Cisplatin[144]
|
|
✓ |
|
|
Cyclosporine[138]
|
|
|
✓ |
|
Danazol[145]
|
✓ |
|
|
|
Dantrolene[146]
|
✓ |
|
|
|
Dapsone[147]
|
✓ |
|
✓ |
|
Disulfiram[148]
|
✓ |
|
|
|
Enalapril[138]
|
✓ |
|
|
|
Erythromycin[149]
|
|
|
✓ |
|
FeSO4
[150]
|
✓ |
|
|
|
Floxuridine[151]
|
✓ |
|
|
|
Gold[135]
|
✓ |
|
|
|
Isoniazid[152]
|
✓ |
|
|
|
Ketoconazole[142]
|
✓ |
|
✓ |
|
Methimazole[153]
|
|
|
✓ |
|
Methotrexate[154]
|
✓ |
✓ |
|
|
Methyldopa[155]
|
✓ |
|
|
|
Nafcillin[135]
|
|
|
✓ |
|
Nevirapine[142]
|
✓ |
|
|
|
Niacin[156]
|
✓ |
|
|
|
Nitrofurantoin[141]
|
✓ |
|
|
|
NSAIDs[157]
[158]
[159]
(diclofenac, naproxen, indomethacin, piroxicam,
etodolac, oxaprozin) |
✓ |
|
✓ (sulindac) |
|
Pemoline[160]
|
✓ |
|
|
|
Penicillins (oxacillin)[149]
|
|
|
✓ |
|
Phenytoin[146]
|
✓ |
|
|
|
Propafenone[142]
|
|
|
|
|
Propylthiouracil[159]
|
✓ |
|
✓ |
|
Quinidine[158]
|
|
|
|
|
Rifampin[161]
|
✓ |
|
✓ |
|
Riluzole[162]
|
✓ |
|
✓ |
|
Steroids, anabolic[163]
|
✓ |
|
|
|
Steroids, oral contraceptives[164]
|
|
|
✓ |
|
Sulfonamides[159]
|
✓ |
|
|
|
Tacrine[165]
|
✓ |
|
|
|
Tamoxifen[142]
|
✓ |
✓ |
✓ |
|
Tetracycline IV[166]
|
✓ |
✓ |
|
|
Ticlopidine[158]
|
|
|
✓ |
|
Tolcapone[167]
|
✓ |
|
|
|
Total parenteral nutrition[168]
|
|
|
✓ |
|
Trazodone[146]
|
✓ |
|
✓ |
|
Valproic acid[169]
|
|
✓ |
|
|
Vitamin A[170]
|
✓ |
✓ |
|
|
Zafirlukast[171]
|
✓ |
|
|
volunteers who underwent radionuclide imaging of the gallbladder before and after
remifentanil infusion. The recovery time for dye to appear in the duodenum after
discontinuation of the infusion was significantly greater than in the control phase
of the study. The authors noted, however, that this delay in recovery of gallbladder
function was shorter than in studies using morphine or meperidine. Finally, no data
support the benefit or detriment of regional versus general anesthesia, nor do data
suggest particular anesthetic agents that should be administered or avoided with
preexisting hepatic dysfunction, with the exception of halothane, as previously discussed.
Patients with more advanced liver disease (e.g., Child-Pugh class
B or C cirrhosis) require an anesthetic plan designed to maximize hepatic oxygen
delivery and prevent and treat the complications of encephalopathy, cerebral edema,
coagulopathy, hemorrhage, and portal hypertension. Hepatic encephalopathy commonly
occurs in patients with chronic liver disease because of the accumulation of circulating
neurotoxins such as unmetabolized ammonia, gut-derived false neurotransmitters, γ-aminobutyric
acid (GABA), and endogenous GABA receptor agonists; altered neurotransmission by
the excitatory neurotransmitter glutamate; or altered cerebral energy homeostasis.
[199]
Clinically, hepatic encephalopathy is manifested
by neuropsychiatric abnormalities ranging from subtle personality changes and cognitive
dysfunction to more advanced depression of consciousness, delirium, and coma. Clinical
signs include abnormalities in psychometric testing and apraxia, as well as the more
severe findings of asterixis, hyperreflexia, and decerebration. When hepatic encephalopathy
occurs with acute liver failure, it often has a rapid onset and is invariably complicated
by cerebral edema. Cerebral edema develops as a result of the osmotic effect of
accumulated glutamine producing astrocyte cell swelling or as a result of cerebral
vasodilation caused by loss of autoregulation, or because of both mechanisms.[200]
These patients invariably require urgent liver transplantation.
A multitude of factors may precipitate hepatic encephalopathy
in patients with chronic liver disease. Potassium levels should be monitored and
hypokalemia treated to decrease the effect of decreased potassium levels on renal
production of ammonia. Arterial pH should be maintained near normal because systemic
alkalemia is also noted to increase the diffusion of ammonia across the blood-brain
barrier, which can potentially worsen the degree of encephalopathy.[199]
Effective circulating blood volume should be maintained and anemia corrected to
maintain hepatic oxygenation and promote the metabolism of circulating toxins. Benzodiazepines
should be used judiciously because they may activate the central GABA-benzodiazepine
receptor ligand and worsen hepatic encephalopathy. In this regard it should be noted
that all psychoactive drugs will have potential deleterious effects on hepatic encephalopathy
by further suppressing a vulnerable central nervous system. Hepatic encephalopathy
is also a recognized complication of the TIPS procedure.[201]
When patients remain obtunded or comatose after anesthesia and
surgery, therapy is often directed toward decreasing ammonia production. Lactulose
is administered either by nasogastric tube or by enema to create an osmotic, cathartic
effect and to lower intestinal pH, thereby decreasing the survival of ammonia-producing
bacteria.[151]
Flumazenil, a specific antagonist
of central benzodiazepine receptors, can improve the level of consciousness in selected
cirrhotic patients with severe hepatic encephalopathy.[202]
Perioperative hemorrhage in patients with significant liver dysfunction
may occur because of bleeding diatheses or the complications of portal hypertension
(or both). Synthetic hepatic function, as measured by the INR, is useful to assess
the severity of the coagulopathy and to gauge therapy, including the subcutaneous
administration of vitamin K, transfusion of fresh frozen plasma, use of recombinant
factor VII or other factors, and plasmapheresis.[192]
In patients with acute liver failure, plasmapheresis may have potential benefit
because it promotes rapid correction of coagulopathy while minimizing volume overload,
but at this time no large prospective controlled studies have validated this practice.
[203]
Hemorrhage may also occur as a result of gastrointestinal bleeding
from rupture of esophageal or gastric varices as a complication of portal hypertension.
Intraoperatively, it is advisable to avoid the placement of a transesophageal echocardiography
probe to avoid initiation of bleeding in patients with esophageal varices. Pharmacologic
therapy for acute esophageal variceal bleeding includes use of the combination of
vasopressin and nitroglycerin, somatostatin, or octreotide, a synthetic analog of
somatostatin.[204]
[205]
Portal hypertension may also lead to the development of ascites.
Ascites occurs with advanced liver disease secondary to renal retention of sodium
and water and localization of this excess fluid in the peritoneal cavity because
of portal hypertension. In addition to the general measures of sodium and water
restriction and diuretic therapy, therapeutic paracentesis may be indicated if a
patient presents for surgery with tense ascites.[206]
Relief of
tense ascites may improve pulmonary gas exchange and reduce the risk of gastric aspiration.
Care should be taken, however, to prevent circulatory collapse by the concomitant
administration of intravenous colloid solutions because intravascular volume re-equilibration
occurs 6 to 8 hours after the removal of a larger volume of ascitic fluid.[204]
Finally, patients with end-stage liver disease are at risk for the development of
either hepatorenal or hepatopulmonary syndrome. Hepatorenal syndrome is a state
of functional renal failure characterized by azotemia, hyperosmolar urine, and urinary
sodium excretion less than 10 mEq/L.[207]
Although
the pathogenesis of this syndrome has not been clearly elucidated, there appears
to be a decrease in renal perfusion pressure related to systemic vasodilation in
combination with a loss of renal autoregulation because of increased sympathetic
activity.[206]
The diagnosis of hepatorenal syndrome
in patients with end-stage liver disease usually signals the need for orthotopic
liver transplantation. In the event that transplantation is not possible, management
is supportive and consists of the use of intravenous volume challenges and large-volume
paracentesis to relieve tense ascites.
The hepatopulmonary syndrome, which is defined by the triad of
end-stage liver disease, increased alveolar-arterial gradient, and intrapulmonary
vascular dilation, is characterized by the clinical features of digital clubbing,
cyanosis, dyspnea, platypnea, and orthodeoxia, in addition to the other clinical
characteristics of portal hypertension. Orthodeoxia, defined as arterial oxygen
desaturation that is more pronounced in the upright position and relieved by recumbency,
is a common phenomenon of hepatopulmonary syndrome.[208]
The pathogenesis of hepatopulmonary syndrome is considered to be primarily related
to intrapulmonary vascular dilation diagnosed by contrast-enhanced echocardiography,
perfusion lung scanning, or pulmonary arteriography. Current pharmacologic therapy
for this disorder is limited; however, orthotopic liver transplantation may lead
to a reversal of these pulmonary changes.
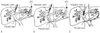 Figure 55-7
Transjugular intrahepatic portal-systemic shunt (TIPS)
procedure. A stent (or stents) is passed through the internal jugular vein over
a wire into the hepatic vein (A); dilated esophageal
varices (EV) are apparent. The wire and stent or stents are then advanced into the
portal vein (B), after which blood can pass through
the portal vein into the hepatic vein and bypass and decompress dilated esophageal
veins (C). (Reproduced with permission from
www.med.umich.edu/1libr/topics/liver09.htm.)
Figure 55-7
Transjugular intrahepatic portal-systemic shunt (TIPS)
procedure. A stent (or stents) is passed through the internal jugular vein over
a wire into the hepatic vein (A); dilated esophageal
varices (EV) are apparent. The wire and stent or stents are then advanced into the
portal vein (B), after which blood can pass through
the portal vein into the hepatic vein and bypass and decompress dilated esophageal
veins (C). (Reproduced with permission from
www.med.umich.edu/1libr/topics/liver09.htm.)
 Figure 55-7
Transjugular intrahepatic portal-systemic shunt (TIPS)
procedure. A stent (or stents) is passed through the internal jugular vein over
a wire into the hepatic vein (A); dilated esophageal
varices (EV) are apparent. The wire and stent or stents are then advanced into the
portal vein (B), after which blood can pass through
the portal vein into the hepatic vein and bypass and decompress dilated esophageal
veins (C). (Reproduced with permission from
www.med.umich.edu/1libr/topics/liver09.htm.)
Figure 55-7
Transjugular intrahepatic portal-systemic shunt (TIPS)
procedure. A stent (or stents) is passed through the internal jugular vein over
a wire into the hepatic vein (A); dilated esophageal
varices (EV) are apparent. The wire and stent or stents are then advanced into the
portal vein (B), after which blood can pass through
the portal vein into the hepatic vein and bypass and decompress dilated esophageal
veins (C). (Reproduced with permission from
www.med.umich.edu/1libr/topics/liver09.htm.)