|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The most appropriate regimen of intraoperative anesthesia and postoperative analgesia for high-risk patients undergoing vascular surgery remains controversial. Competing concerns regarding the quality and escalating costs of perioperative care have challenged all clinicians over the past decade to establish practice standards that are safe and efficient.[498] Postoperative complications after vascular surgery are common and adversely impact clinical outcome and resource use.[499] Improvement in clinical outcome and reduced use of medical resources in patients undergoing vascular surgery may result from the use of one particular regimen of anesthesia and analgesia over another.[118] [500] If such improvement can be achieved, the selection of the most appropriate anesthetic and analgesic regimen would then be of great benefit to the patients, providers, payers, and society.
The question of whether regional or general anesthesia is preferable for vascular surgery has been debated for years, but only in the past decade have well-designed, prospective, randomized trials been completed. Earlier nonrandomized trials were prone to significant bias because many clinicians had the unfounded belief that regional anesthesia was safer for patients with advanced cardiac or pulmonary disease. Even the prospective studies must be cautiously interpreted as many suffer from deficiencies of design and methodology, including nonuniform patient populations, [118] [298] [500] lack of standardization or control of perioperative treatments,[118] [295] [296] [298] [301] [302] [500] [501] use of nonequivalent modalities for post-operative pain relief,[118] [295] [298] [301] [500] [501] and possible investigator bias.[79] [118] [295] [296] [297] [298] [301] [302] [500] [501] Many clinical trials have attempted to optimize the delivery and management of the anesthetic techniques, which may mask the true risks of the anesthetic. An example of this is the strict hemodynamic control, transfusion thresholds, and postoperative analgesia regimens that my colleagues and I have used in our clinical trials.[79] [132] The physician must also appreciate that the overall complication rates reported in clinical trials may seem high, but this may be explained by the aggressive surveillance commonly used in clinical trials. In general, it is often best to choose the anesthetic and analgesic techniques that are most familiar to a particular institution because, for example, unfamiliarity and mismanagement of epidural catheters can cause serious complications. It is my personal hypothesis that overall optimization of perioperative care, rather than anesthetic or analgesic selection, is the most important factor in improving outcome with vascular surgery.
Occasionally, surgical procedures, such as embolectomy and femoral pseudoaneurysm repair, can be completed with the patient under local anesthesia with intravenous sedation. Because these procedures may progress to more invasive arterial reconstruction, a regional or general anesthesia should be considered before the procedure begins to avoid an unplanned conversion to general anesthesia.
In some conditions, one anesthetic technique (regional or general) is preferable to the other. Anticoagulant and antithrombolytic therapy is common in the vascular surgery population and often precludes the use of neuraxial techniques. Symptomatic bleeding within the neuraxis (spinal or epidural hematoma) is a potentially devastating complication that can lead to permanent neurologic injury. I view preoperative anticoagulation with heparin, warfarin, or thrombolytic drugs as a contraindication to the use of spinal and epidural anesthesia. For patients recently discontinued from such agents, very careful
Given the relative risks associated with neuraxial anesthesia in patients receiving anticoagulant or antithrombolytic therapy, some clinicians advocate the broader use of peripheral nerve blocks, such as sciatic, femoral, popliteal, and ankle (see Chapter 44 ). Continuous catheter techniques can be used to provide anesthesia and postoperative analgesia. High-resolution ultrasound imaging of neural structures, percutaneous electrode guidance, and use of stimulating catheters have been introduced into clinical practice. Peripheral nerve blocks probably are associated with fewer systemic and neuraxial side effects, but little clinical information is available in the vascular surgical population. Because of the high volume of local anesthetics frequently used for peripheral nerve blocks, the issue of systemic toxicity must be considered. I advise caution with the use of peripheral nerve block in the anticoagulated patient, particularly when the neural structures are deep or located close to vascular structures.
Because regional anesthesia does not require airway instrumentation, neuromuscular blocking agents, or volatile agents, it has traditionally been a prevailing belief that regional anesthesia is preferable in patients with significant pulmonary disease. Although it is true that instrumentation of the airway may precipitate bronchospasm or increase the risk of nosocomial infection, general anesthesia with endotracheal intubation does allow for complete airway and ventilation control, the ability to effectively administer inhaled bronchodilators, and the ability to easily suction airway secretions. A reduction in time to extubation after aortic surgery is a fairly consistent theme with regional anesthesia and analgesia, but this does not appear to have any impact on clinically relevant pulmonary outcomes.[132] [295] [298] [302]
Epidural analgesia has been championed over parenteral opioid analgesia as a means to optimize postoperative pulmonary function by improving pain control and respiratory muscle function. Although epidural analgesia can provide excellent postoperative pain control and may improve postoperative lung function (i.e., increased tidal volume and vital capacity), clinical studies do not support a consistent finding of improved pulmonary outcomes.[510] Overall, there is little evidence from well-designed clinical studies to demonstrate improved pulmonary outcome with regional anesthesia and analgesia.[511] Because postoperative maneuvers to increase mean lung volumes are of proven benefit in preventing postoperative pulmonary complications, it has been recommended that maneuvers to encourage deep breathing, such as deep breathing exercises, incentive spirometry, and chest physiotherapy, should be the focus of preventive efforts.[511]
Cardiac morbidity is the most common cause of death in patients undergoing surgery, and the incidence of perioperative cardiac morbidity is 10 times greater in vascular surgery patients than in nonvascular surgery patients.[26] Eleven prospective randomized trials evaluating the effects of regional versus nonregional anesthesia with or without analgesia in vascular surgical patients have reported on cardiac outcomes and death. Three studies (Cook and colleagues,[512] Christopherson and coworkers,[79] and Bode and associates [501] ) compared pure regional (spinal or epidural) versus general anesthetic techniques in lower extremity vascular patients. Tuman and colleagues[500] compared combined epidural plus general versus general anesthetics in aortic and lower extremity surgical patients. Six studies (Baron and coworkers,[295] Davies and associates,[296] Garnett and colleagues,[301] Bois and coworkers,[297] Boylan and associates, [302] and Norris and colleagues[132] ) compared epidural versus nonepidural anesthetic and/or analgesic techniques in aortic surgical patients. Fleron and coworkers[300] compared intrathecal opioid versus intravenous analgesia in aortic surgical patients. Only the study by Norris and associates[132] had a double-blind design. The results of these studies, involving more than 1500 patients, are shown in Table 52-12 . In summary, no study demonstrated any difference in outcome with regard to mortality, MI, myocardial ischemia, or congestive heart failure. Only the study by Tuman and colleagues[500] reported a difference in cardiac outcome. Outcome was significantly improved by epidural anesthesia or analgesia, but only when more subtle outcomes (dysrhythmias) were included. Christopherson and coworkers[79] (i.e., PIRAT study) and Norris and associates[132] (i.e., PIRAT II study) found no difference in cardiac events or myocardial ischemia as detected by continuous Holter monitor over a 3-day postoperative period. In these studies, strict intraoperative and postoperative protocols were used to guide and optimize perioperative management
|
|
|
Death | MI | Myocardial Ischemia | CHF | Graft Occlusion |
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | No. of Patients | RA | GA | RA | GA | RA | GA | RA | GA | RA | GA | Comments |
| Cook et al.[512] | 101 | 2% | 6% | 4% | 2% |
|
|
|
|
|
|
Spinal anesthesia, lower extremity surgery |
| Tuman et al.[500] | 80 | 0% | 0% | 0% | 8% |
|
|
5% | 10% | 3% | 20% | Aortic and lower extremity surgery |
| Baron et al.[295] | 167 | 4% | 5% | 6% | 6% | 20% | 19% | 6% | 8% |
|
|
Aortic surgery |
| Christopherson et al.[79] | 100 | 2% | 2% | 4% | 4% | 35% | 45% |
|
|
4% | 22% | Lower extremity surgery |
| Davies et al.[296] | 50 | 8% | 4% | 8% | 4% |
|
|
12% | 8% |
|
|
Aortic surgery |
| Garnett et al.[301] | 99 | 0% | 4% | 6% | 10% | 58% | 51% | 6% | 10% |
|
|
Aortic surgery |
| Bode et al.[501] [513] | 423 | 3% | 3% | 5% | 4% |
|
|
10% | 9% | 6% | 4% | Spinal and epidural anesthesia, lower extremity surgery |
| Bois et al.[297] | 114 | 2% | 2% | 4% | 8% | 18% | 19% | 5% | 0% |
|
|
Aortic surgery |
| Boylan et al.[302] | 40 | 0% | 0% | 5% | 5% | 32% | 38% | 11% | 5% |
|
|
Aortic surgery |
| Norris et al.132a | 168 | 5% | 5% | 4% | 0% | 16% | 17% | 1% | 0% |
|
|
Aortic surgery, double-blind study |
| *Any epidural use included in regional anesthesia group. | ||||||||||||
| CHF, congestive heart failure; GA, general anesthesia; MI, myocardial infarction; RA, regional anesthesia. | ||||||||||||
One of the most interesting and clinically significant findings in these randomized trials is the beneficial effect of regional anesthesia on lower extremity graft patency in the postoperative period. Two of the studies (Tuman and colleagues[500] and Christopherson and coworkers [79] ) reported a fivefold greater incidence of graft occlusion after general (relative to regional) anesthesia. Most graft occlusions occurred in the first 1 to 3 days after surgery, after which the established difference in the incidence of graft occlusion between anesthetic techniques was maintained over time (6 weeks and beyond) ( Fig. 52-19 ). This time course suggests that anesthetic technique may have played a role in graft occlusion. Bode and associates[513] reported an overall very low incidence of graft occlusion, but differences in hemodynamic management, surgical technique, and the patient population may explain these findings. For example, intraoperative intravascular angioscopy was used to inspect the grafts to confirm patency before completion of surgery, and all patients were cared for in an intensive care setting for 48 hours after surgery. Optimization of care with respect to graft patency may negate any beneficial effect of regional techniques. It is also important to keep in mind that
 Figure 52-19
Cumulative probability of reoperation for regrafting,
thrombectomy, or amputation over a 6-week follow-up period. Reoperation was significantly
more common after general anesthesia than after epidural anesthesia. (From
Christopherson R, Beattie C, Fran SM, et al: Perioperative morbidity in patients
randomized to epidural or general anesthesia for lower extremity vascular surgery.
Perioperative Ischemia Randomized Anesthesia Trial Study Group. Anesthesiology
79:422–434, 1993.)
Figure 52-19
Cumulative probability of reoperation for regrafting,
thrombectomy, or amputation over a 6-week follow-up period. Reoperation was significantly
more common after general anesthesia than after epidural anesthesia. (From
Christopherson R, Beattie C, Fran SM, et al: Perioperative morbidity in patients
randomized to epidural or general anesthesia for lower extremity vascular surgery.
Perioperative Ischemia Randomized Anesthesia Trial Study Group. Anesthesiology
79:422–434, 1993.)
The proposed mechanism for the benefit of regional anesthesia is the effects of anesthetic technique on coagulation. General anesthesia is associated with a hypercoagulable state in the early postoperative period, whereas regional anesthesia attenuates this effect.[515] Tuman and colleagues[500] demonstrated this by thromboelastography and Rosenfeld and coworkers[515] by increased plasminogen activator inhibitor and fibrinogen levels. It appears that fibrinolysis is decreased after general anesthesia and is normal after regional anesthesia. These findings may be related to attenuation of the surgical stress response with regional anesthesia because there appears to be a link between stress, catecholamines, and acute-phase reactants, such as plasminogen activator inhibitor and fibrinogen.[516] [517] Platelet reactivity is also enhanced in the presence of a stress response.[517] Another important mechanism for increased lower extremity graft patency with regional anesthesia may be the increased lower extremity blood flow associated with sympathectomy.[518]
In the postoperative period, Breslow and associates[128] demonstrated differences in adrenergic responses with general and with regional anesthesia ( Fig. 52-20 ). Epinephrine and norepinephrine are increased after general anesthesia relative to regional anesthesia. The cortisol response after general anesthesia is also greater than that after regional anesthesia.[128] This "stress response" is associated with increased blood pressure and hemodynamic liability during the intraoperative and early postoperative periods after general anesthesia compared with regional anesthesia. [79] [519] When the hemodynamic parameters are controlled pharmacologically, however, there is no difference related to anesthetic technique in myocardial ischemia or cardiac morbidity. [79] [132] [501]
Postoperative pain is recognized as one of the many factors contributing to the surgical stress response. In studies comparing epidural analgesia versus parenteral opioid analgesia for postoperative pain control after major surgery, improved pain control with epidural techniques is often reported. A meta-analysis review supports the view that epidural analgesia provides better postoperative analgesia than parenteral opioids.[520] However, the historical studies that form the basis of the meta-analysis review have often neglected to control, specify, and most importantly, optimize treatment in the nonepidural arms of their studies. Unfortunately, this issue remains a significant limitation in more recent trials.[298] [521] My colleagues and I believe that epidural analgesic techniques will continue to outperform suboptimal nonepidural analgesic techniques. We believe that intravenous patient-controlled analgesia is the optimal mode of delivery for opioid analgesia and should be used as the nonepidural arm of all postoperative pain studies. Postoperative epidural analgesia does not consistently outperform intravenous patient-controlled opioid analgesia.[522] Of particular note, patient-controlled epidural analgesia outperforms intermittent-bolus and continuous-infusion epidural analgesia.[523] Thus, mode of
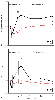 Figure 52-20
Plasma norepinephrine (A)
and epinephrine (B) concentrations before induction
of anesthesia (P), at skin closure (C), and at 1, 6, 12, and 18 hours after lower
extremity revascularization. GA, general anesthesia with postoperative parenteral
morphine by patient-controlled analgesia; RA, regional (epidural) anesthesia with
postoperative epidural analgesia by patient-controlled analgesia. (Adapted
from Breslow MJ, Parker SD, Frank SM, et al: Determinants of catecholamine and cortisol
responses to lower extremity revascularization. The PIRAT Study Group. Anesthesiology
79:1202–1209, 1993.)
Figure 52-20
Plasma norepinephrine (A)
and epinephrine (B) concentrations before induction
of anesthesia (P), at skin closure (C), and at 1, 6, 12, and 18 hours after lower
extremity revascularization. GA, general anesthesia with postoperative parenteral
morphine by patient-controlled analgesia; RA, regional (epidural) anesthesia with
postoperative epidural analgesia by patient-controlled analgesia. (Adapted
from Breslow MJ, Parker SD, Frank SM, et al: Determinants of catecholamine and cortisol
responses to lower extremity revascularization. The PIRAT Study Group. Anesthesiology
79:1202–1209, 1993.)
In the only double-blinded trial in vascular surgery patients, Norris and colleagues[132] reported no difference
|
|
|
|
|
|
|
|
|
|
|
|
|