|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Thoracic and thoracoabdominal aortic surgery can be performed without extracorporeal support. Large series of the "clamp-and-sew" technique have been published with relatively favorable outcomes, but these cases are from institutions with the greatest clinical experience and the shortest cross-clamp times.[314] [328] [331] Other than the location and the extent of the aneurysm, the duration of cross-clamp on the aorta is the single most important determinant of paraplegia and renal failure when bypass is not employed. Clamp times of less than 20 to 30 minutes are associated with almost no paraplegia.[314] [355] When clamp times are between 30 and 60 minutes (the vulnerable interval), the incidence of paraplegia increases from approximately 10% to 90% as time progresses.[356] [357] Because clamp times are typically in this range or longer, efforts are usually made to perfuse the distal aorta to protect the viscera, kidneys, and spinal cord from ischemic injury.
When the simple clamp-and-sew technique is used, application of the aortic cross-clamp results in significant proximal hypertension, which requires active pharmacologic intervention. Management strategies have been discussed previously in the context of abdominal aortic reconstruction. Briefly, sodium nitroprusside [217] and isoflurane[225] have been used successfully to control the proximal hypertension associated with high aortic cross-clamping. Isoflurane is best reserved for those patients with good myocardial function. Vasodilators, such as sodium nitroprusside, must be used with caution because they can result in significant overperfusion of the body proximal to the clamp and very low pressures distally.[229] Nitroglycerin can be used to normalize preload and cardiac filling and to reduce ventricular wall tension. Although nitroglycerin does not control proximal hypertension well as a single agent, it is very helpful when used in combination with sodium nitroprusside. The management of aortic unclamping has been previously discussed.
Maintaining lower body perfusion with the use of retrograde aortic perfusion reduces ischemic injury and improves outcome, provided that the pressure is high enough to perfuse the organs. The simplest method of providing distal aortic perfusion is a passive conduit or shunt. The heparin-bonded Gott shunt[358] was developed to avoid the need for systemic heparinization and is used to divert flow passively from the left ventricle or proximal descending thoracic aorta to the distal aorta. Some center place a temporary axillary-to-femoral artery graft to function as a shunt during aortic cross-clamping.
Partial bypass, also referred to as left heart bypass or left atrial-to-femoral bypass, is the most commonly used distal aortic perfusion technique ( Fig. 52-16 ). This technique allows the adjustment of blood flow and usually draws blood from the left atrium and returns blood into the left femoral artery. A centrifugal pump is used (Biomedicus, Eden Prairie, MN), and there is no need for full-dose systemic heparin because the circuit is heparin-coated. The
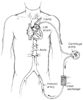 Figure 52-16
Diagram of a left atrial-to-femoral bypass. The left
atrium and the left femoral artery are cannulated, and a centrifugal pump is used
with heparin-coated tubing. A heat exchanger may be added into the circuit for cooling
and rewarming.
Figure 52-16
Diagram of a left atrial-to-femoral bypass. The left
atrium and the left femoral artery are cannulated, and a centrifugal pump is used
with heparin-coated tubing. A heat exchanger may be added into the circuit for cooling
and rewarming.
During left heart bypass, it is essential to monitor arterial blood pressure above and below the aortic cross-clamps. I simultaneously display the radial and femoral artery pressures and aim for a mean arterial pressure of 80 to 100 mm Hg above the cross-clamp and at least 60 mm Hg below the cross-clamp.[354] Careful control of
Complex aneurysms involving the aortic arch often require elective cardiopulmonary bypass with an interval of deep hypothermic circulatory arrest (DHCA), because cerebral blood flow is transiently interrupted during surgery (see Chapter 50 ). This can be accomplished by cannulation of the femoral artery and the femoral vein (i.e., femoral-femoral bypass). Some centers also use anterograde (i.e., innominate artery) or retrograde (i.e., internal jugular vein) selective cerebral perfusion with cold oxygenated blood to extend the safe maximum duration of DHCA. [360] Without this technique, 45 to 60 minutes is thought to be the safe limit of DHCA, but 90 minutes has been reported with selective cerebral perfusion.[361] DHCA may also be necessary for patients with previous aortic arch repairs because adhesions and scarring make application of the proximal aortic cross-clamp difficult or impossible during TAA repair.[362] DHCA eliminates the need for proximal aortic clamping and allows a bloodless field for the proximal aortic anastomosis. After the completion of the proximal anastomosis and intercostal artery-to-graft anastomoses under DHCA, the graft is cannulated and bypass flow is reestablished into the upper aorta. Roughly one third of the arterial bypass flow is directed into the upper aorta and two thirds into the distal aorta. During a period of hypothermic low bypass flow, the distal anastomoses are completed and then rewarming is initiated.
There is no single best anesthetic technique for TAA repair. Usually, a balanced anesthetic is given with a combination of an opioid, a low-dose potent inhalation agent, a benzodiazepine, and a long-acting muscle relaxant. A total intravenous technique may be optimal if transcranial MEP monitoring is used. The induction should be slow and controlled without hypotension or hypertension because stress on the aneurysm can cause rupture. Heart rate should be maintained near baseline because myocardial ischemia is often heart rate related. Postoperative analgesia is sometimes managed with an epidural catheter.
Paraplegia is a devastating complication of aortic surgery. The incidence of paraplegia is reported to be 0.5% to 1.5% for coarctation repair, zero to 10% for thoracic aneurysm repair, 10% to 20% for thoracoabdominal repair, and as high as 40% for extensive dissecting TAA repair.[321] [363] [364] [365] [366] [367]
The spinal cord receives its blood supply from two posterior arteries and one anterior spinal artery ( Fig. 52-17 ). The anterior spinal artery, which supplies the motor tracts in the spinal cord, is formed by two branches of the intracranial portion of the vertebral arteries.[368] The upper cervical segment of the spinal cord receives most of its blood flow from the vertebrals.[369] [370] The thoracic portion
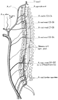 Figure 52-17
Diagram of the blood supply to the spinal cord shows
the anterior and posterior radiculomedullary branches in a lateral view. The primary
blood supply to the thoracolumbar portion of the spinal cord is derived from the
artery of Adamkiewicz; its origin varies, but it usually branches off the aorta in
the T9 to T12 region. (From Djindjian R: Arteriography of the spinal cord.
Am J Roentgenol Radium Ther Nucl Med 107:461–478, 1969.)
Figure 52-17
Diagram of the blood supply to the spinal cord shows
the anterior and posterior radiculomedullary branches in a lateral view. The primary
blood supply to the thoracolumbar portion of the spinal cord is derived from the
artery of Adamkiewicz; its origin varies, but it usually branches off the aorta in
the T9 to T12 region. (From Djindjian R: Arteriography of the spinal cord.
Am J Roentgenol Radium Ther Nucl Med 107:461–478, 1969.)
Various methods have been used to prevent ischemic injury to the spinal cord. Distal perfusion with extracorporeal support has been shown to reduce the incidence of paraplegia.[375] [376] Some form of bypass is likely to be beneficial when the anticipated cross-clamp time is greater than 30 minutes, but is probably not beneficial when cross-clamp time is less than 20 to 30 minutes. CSF drainage is frequently used to improve spinal cord perfusion during TAA repair, and is often used in combination with distal aortic perfusion.[327] [377] Spinal cord perfusion pressure is defined as distal mean aortic pressure minus CSF pressure or central venous pressure, whichever is greatest. Autoregulation of spinal cord blood flow is similar to cerebral autoregulation, and blood flow is relatively constant over the range of 50 to 125 mm Hg.[378] During hypoxia or hypercarbia, autoregulation is lost, and flow becomes linearly related to perfusion pressure. Significant flow may remain even at very low perfusion pressures. Drainage of CSF is thought to be important because CSF pressure often increases (by 10 to 15 mm Hg) with application of thoracic aortic cross-clamps.[379] The increase in CSF pressure reduces spinal cord perfusion pressure and increases the likelihood of ischemic spinal cord injury.
Despite evidence from animal studies that CSF drainage protects the spinal cord,[380] [381] the clinical use of this technique is controversial. One randomized trial reported a reduced incidence of paraplegia,[382] but another reported no benefit.[383] Most of the evidence in support of CSF drainage is from nonrandomized historical cohort studies in which the technique is used in combination with other adjuncts, such as intrathecal papaverine [382] [384] and hypothermic partial bypass.[327] [332] [354] [377] Coselli and colleagues[385] offered the strongest evidence supporting the efficacy of CSF drainage. They conducted a prospective, randomized clinical trial to evaluate the impact of CSF drainage on the incidence of spinal cord injury after Crawford type I and type II TAA repair. CSF drainage resulted in an 80% reduction in the relative risk of postoperative deficit. Nine patients (13%) in the control group had paraplegia or paraparesis compared with only two patients (2.6%) in the CSF drainage group. Left heart bypass, moderate heparinization, permissive mild hypothermia, and reimplantation of patent intercostal and lumbar arteries were performed in both treatment groups. The target CSF pressure was 10 mm Hg. CSF drainage has also been shown (in case reports) to reverse delayed-onset neurologic deficit after open[386] and endovascular[387] TAA repair.
Although CSF drainage is widely used during TAA repair, the technique is not without risks. Potential complications include headache, meningitis, chronic CSF leakage, spinal or epidural hematoma, and subdural hematoma. The possibility of intraspinal pathology should be considered with any postoperative lower extremity neurologic deficit.[388] A retrospective review of 230 patients who underwent TAA repair with CSF drainage at my institution reported eight subdural hematomas (3.5%).[389] High volume CSF drainage was identified as a risk factor for its occurrence. Six patients had subdural hematomas detected during hospitalization, with an associated mortality of 67%. Two patients were seen in a delayed fashion, and both required epidural blood patch to control chronic CSF leakage.
Hypothermia is probably the most reliable method of neuroprotection from ischemic injury. By reducing oxygen requirements by approximately 5% for each degree centigrade, a twofold prolongation of tolerated cross-clamp time is achieved by cooling even to mild hypothermia (34°C).[390] Because the reduction in metabolic rate is linearly related to temperature, moderate or profound hypothermia provides even greater protection. Both systemic and local spinal cord cooling have been shown to be beneficial. Systemic hypothermia can been achieved with full cardiopulmonary bypass (with or without DHCA)[391] or partial bypass.[354] [392] Cooling to 30°C to 32°C with a left atrial-to-femoral bypass (LA-FA) bypass in combination with CSF drainage was associated with no permanent neurologic sequelae in a series of 20 patients, despite a relatively long average cross-clamp time (approximately 70 minutes).[354] My colleagues and I have since used this technique in more than 200 patients cooled to 32°C, with a 5% incidence of paraplegia. Although some risk is incurred when a beating heart is subjected to moderate hypothermia, the benefits appear to outweigh the risks. Supraventricular and ventricular dysrhythmias respond well to cardioversion and to mild warming to 33°C to 34°C. Localized cooling of the spinal cord by cold perfusion of the GRA with blood[393] or crystalloid[394] provides significant protection during spinal ischemia in animal models. Local cooling has been shown to be beneficial in humans who received epidural infusions of 4°C saline.[395] Even if active cooling is not used, it is advantageous to allow patients to passively cool to 33°C to 34°C during TAA repair. With passive cooling, the challenge is rewarming after the surgical repair. This is most easily accomplished by use of a forced-air blanket over the upper body. The lower body should not be actively warmed, because warming ischemic tissue increases metabolic requirements, acidosis, and ischemic injury.
A variety of drugs have been the focus of laboratory and clinical investigation in an attempt to reduce the incidence of ischemic spinal cord injury. Barbiturates have been shown to provide significant protection.[396] [397] Corticosteroids have been shown to provide protection in dogs,[398] but were beneficial in humans only when they were combined with CSF drainage.[399] Calcium channel blockers were protective against spinal cord ischemia in some studies, [400] [401] but not in others.[402] [403] N-methyl-D-aspartame receptor antagonists have been investigated because ischemic injury appears to be related to increased
Preoperative spinal cord angiography in patients with TAA has been reported by several groups.[412] [413] [414] The rational for this highly invasive angiographic procedure is that precise identification of intercostal arteries giving rise to the GRA allows focused reimplantation of these vessels during surgical repair and helps prevent spinal cord injury. Selective intercostal angiography identifies the GRA when an intercostal branch is found making a cephalad hairpin turn to enter the spinal canal and supply a midline longitudinal artery ( Fig. 52-18 ). The GRA can be identified in 43% to 86% of patients studied.[412] [413] One report found that even in patients with an identified and reimplanted GRA, spinal cord injury could not always be prevented. [414] They concluded that spinal cord angiography has no impact on neurologic outcome after TAA repair.
One study[412] reported no improvement in overall neurologic outcome with preoperative spinal cord angiography but offered important insight regarding type of aneurysm, GRA identification, and neurologic outcome. In patients undergoing TAA repair for extensive degenerative aneurysms, spinal cord injury occurred in 0 (0%) of 45 patients versus 10 (12%) of 81 patients with and without an identified GRA, respectively. In contrast, the identification of the GRA was not helpful in the case of chronic expanding aortic dissection, with 3 (15%) of 20 patients versus 3 (6%) of 49 patients developing spinal cord injury with and without an identified GRA, respectively. The investigators hypothesized that mural thrombus in degenerative aneurysms results in the occlusion of many intercostal arteries and favors the development of extensive paravertebral collateral channels (see Fig. 52-18 ). Identification of a GRA allows focused reimplantation with uniform success. In patients with chronic dissection, most intercostal arteries are patent, collateralization is minimal, and reimplantation of one or two intercostal arteries may be insufficient to supply blood flow to the spinal cord.
Renal failure after TAA repair results from preexisting renal dysfunction, ischemia during cross-clamp, thrombotic or embolic interruption in renal blood flow, and hypovolemia and hypotension. Approximately 6% of patients require postoperative dialysis, even in centers with the most clinical experience.[326] [336] The associated mortality can be high.[336] The primary predictor of postoperative
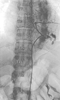 Figure 52-18
Spinal cord angiogram of an extensive degenerative thoracoabdominal
aortic aneurysm. Selective injection of the intercostal artery at T8 (arrow)
demonstrates the GRA and extensive paravertebral collateralization.
Figure 52-18
Spinal cord angiogram of an extensive degenerative thoracoabdominal
aortic aneurysm. Selective injection of the intercostal artery at T8 (arrow)
demonstrates the GRA and extensive paravertebral collateralization.
Coagulopathy is a frequent complication during TAA repair. A dilutional coagulopathy develops during massive transfusion in which platelets become deficient after approximately one blood volume of replacement. At somewhere between one and two blood volumes of replacement, coagulation factors are diluted to levels low enough to increase bleeding. Other contributing factors are residual heparin, ischemia to the liver where most coagulations factors are produced, and persistent hypothermia after weaning from bypass. With the early use of fresh frozen plasma and platelets, severe coagulopathy can often be avoided. The prothrombin time, partial thromboplastin time, fibrinogen, and platelet counts should be measured frequently. Cryoprecipitate may be necessary to correct coagulopathy, especially when the prothrombin time and partial thromboplastin time are prolonged and hypervolemia prevents the administration of significant volumes of fresh frozen plasma. When coagulopathy persists despite these efforts, epsilon-aminocaproic acid is beneficial as antifibrinolytic therapy, and desmopressin can be given to increase circulating levels of von Willebrand factor and factor VIII. Normothermia should be achieved by complete rewarming before separation from bypass, increasing the ambient temperature after separation from bypass, and by forced air warming over the upper body skin surface. Arterial blood gases and electrolyte levels should be measured frequently. Sodium bicarbonate should be given to treat the metabolic acidosis that occurs during and after cross-clamping. Hyperkalemia should be aggressively treated, especially in oliguric or anuric patients. Calcium chloride and sodium bicarbonate are the primary acute treatments for hyperkalemia.
|
|
|
|
|
|
|
|
|
|
|
|
|