THORACOABDOMINAL AORTIC ANEURYSM SURGERY
Thoracic aortic surgery is required for a spectrum of disease,
including degenerative aneurysm, acute and chronic dissection, pseudoaneurysm, coarctation,
and traumatic aortic tear. Thoracoabdominal aortic aneurysm (TAA) repair is often
regarded as the most challenging surgical case in terms of overall anesthetic and
perioperative management. Since the first TAA repair in 1955,[313]
tremendous advances have been made in the field. These advances have led to significant
reductions in operative mortality and perioperative complications. However, even
in centers where numerous procedures are performed, morbidity and mortality rates
are high, especially for patients with dissecting or ruptured aneurysms. To successfully
care for these patients, the anesthesiologist must be knowledgeable in the areas
of one-lung ventilation; extracorporeal circulatory support, including circulatory
arrest; renal and spinal cord protection; induced hypothermia; invasive hemodynamic
monitoring, including TEE; massive transfusion; and management of coagulopathy.
Intraoperative management requires a team effort, with intimate cooperation among
surgeons, anesthesiologists, perfusionists, nurses, and electrophysiologic monitoring
staff. Endovascular repair of lesions that affect the descending thoracic and thoracoabdominal
aorta is evolving, but much more slowly than with infrarenal aortic aneurysm. However,
accumulating experience with stent graft repair of thoracic aortic aneurysm, dissection,
and traumatic tear indicates that it will likely be an effective alternative to open
repair for select patients.
Etiology and Classification
Aneurysms of the thoracoabdominal aorta occur primarily because
of atherosclerotic degenerative disease (80%) and chronic aortic dissection (17%).
[314]
The remainder are caused by trauma, connective
tissue diseases involving the aortic wall from conditions such as Marfan's syndrome,
cystic medial degeneration, Takayasu's arteritis, or syphilitic aortitis.[315]
The true incidence of TAA is unknown, but population studies suggest a prevalence
much less than infrarenal abdominal aortic aneurysm. Degenerative and dissecting
TAAs differ in their associated risk factors, extent of aortic involvement, and natural
history.[316]
[317]
Complete characterization of each TAA is required to formulate a comprehensive treatment
plan. Development of degenerative and dissecting TAA is ultimately related to weakening
of the aortic wall. Although the natural history of TAA without surgery is uncertain,
enlargement tends to be progressive and nonoperative management is generally associated
with a poor prognosis. With progressive enlargement, the nutritional blood flow
to the aorta is compromised. The increasing diameter is associated with increased
wall tension, even when arterial pressure is constant (i.e., law of LaPlace). The
high incidence of associated systemic hypertension enhances aneurysm enlargement.
Degenerative and dissecting TAAs are symptomatic at the time of
presentation in 57% and 85% of patients, respectively.[316]
[317]
The most common presenting complaint
is back pain. Additional symptoms can be caused by compression of organs or structures
adjacent to the aneurysm. Aortic rupture, as a manifestation of TAA, occurs with
equal frequency (9%) in degenerative and dissecting aneurysms.[316]
[317]
Rupture of the thoracic and abdominal segments
occurs with equal frequency.[318]
Rupture occurs
primarily in patients with aneurysms larger than 5 cm.[319]
Surgical repair is usually recommended when aneurysm diameter exceeds 6 cm.[320]
In addition to cause, aneurysms of the thoracoabdominal aorta
may be classified according to their anatomic location. In 1986, Crawford and colleagues,
[314]
recognizing the correlation between aneurysm
extent and clinical outcome, proposed a classification based on extent of aortic
involvement ( Fig. 52-14
).
The Crawford classification defines aneurysms as extent types I, II, III, and IV.
Type I aneurysms involve all or most of the descending thoracic aorta and the upper
abdominal aorta. Type II aneurysms involve all or most of the descending thoracic
aorta and all or most of the abdominal aorta. Type III aneurysms involve the lower
portion of the descending thoracic aorta and most of the abdominal aorta. Type IV
aneurysms involve all or most of the abdominal aorta, including the visceral segment.
Types II and III are most difficult to repair because they involve the thoracic
and abdominal segments of the aorta. The Crawford classification is appropriately
applied to aneurysms of all causes (degenerative and dissecting). Patients with
Crawford type II aneurysms are at greatest risk for paraplegia and renal failure
from ischemia to the spinal cord and kidneys during cross-clamp. Even with extracorporeal
circulatory support, there is an obligatory period when blood flow to these organs
is interrupted because the origin of the blood flow is between the cross-clamps.
For this reason, protective measures to prevent ischemic injury are important in
reducing morbidity.
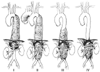 Figure 52-14
The Crawford classification of thoracoabdominal aortic
aneurysms is defined by anatomic location and the extent of involvement. Type I
aneurysms involve all or most of the descending thoracic aorta and the upper abdominal
aorta; type II aneurysms involve all or most of the descending thoracic aorta and
all or most of the abdominal aorta; type III aneurysms involve the lower portion
of the descending thoracic aorta and most of the abdominal aorta; and type IV aneurysms
involve all or most of the abdominal aorta, including the visceral segment. (Adapted
from Crawford ES: Thoracoabdominal and suprarenal abdominal aortic aneurysm. In
Ernst CB, Stanley JC [eds]: Current Therapy in Vascular Surgery. Philadelphia,
Decker, 1987, pp 96–98.)
Figure 52-14
The Crawford classification of thoracoabdominal aortic
aneurysms is defined by anatomic location and the extent of involvement. Type I
aneurysms involve all or most of the descending thoracic aorta and the upper abdominal
aorta; type II aneurysms involve all or most of the descending thoracic aorta and
all or most of the abdominal aorta; type III aneurysms involve the lower portion
of the descending thoracic aorta and most of the abdominal aorta; and type IV aneurysms
involve all or most of the abdominal aorta, including the visceral segment. (Adapted
from Crawford ES: Thoracoabdominal and suprarenal abdominal aortic aneurysm. In
Ernst CB, Stanley JC [eds]: Current Therapy in Vascular Surgery. Philadelphia,
Decker, 1987, pp 96–98.)
Aortic dissection, with or without aneurysm formation, has likewise
been classified based on extent of aortic involvement. The most widely used classification,
proposed by DeBakey and colleagues, defines aortic dissection as types I, II, and
III ( Fig. 52-15
).[321]
Type I aneurysms begin in the ascending aorta and extend throughout the entire aorta.
These lesions are usually repaired by use of a two-stage approach, with the first
procedure on the ascending aorta and aortic arch and the second procedure on the
descending thoracic aorta. Type II aneurysms are confined to the ascending aorta.
Types I and II often involve the aortic valve, causing aortic regurgitation, and
sometimes involve the ostia of the coronary arteries. Type III aneurysms begin just
distal to the left subclavian artery and extend to the diaphragm (type IIIA) or to
the aortoiliac bifurcation (type IIIB). Another commonly used classification of
aortic dissection is the Stanford classification.[322]
This more simplified classification divides aortic dissection into those that involve
the ascending aorta (Stanford type A) and those that do not involve the ascending
aorta (Stanford type B). Aortic dissection is also classified by duration, with
those less than 2 weeks classified as acute and those greater than 2 weeks classified
as chronic. This classification has very significant mortality implications, with
much higher mortality in the acute phase.
Acute aortic dissection involving the ascending aorta (DeBakey
type I and II, Stanford type A) is a surgical emergency and requires immediate cardiac
surgical repair[323]
(see Chapter
50
). Acute dissections involving the descending aorta (DeBakey type III,
Stanford type B) are most often treated conservatively (i.e, blood pressure and pain
control) because surgical repair has no proven benefit over medical or interventional
treatment in stable patients.[324]
Early surgical
intervention may be required for a variety of reasons, including aneurysmal formation,
impending rupture, leg, renal, or visceral ischemia and
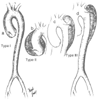 Figure 52-15
The DeBakey classification of dissecting aneurysms of
the aorta. Type I has an intimal tear in the ascending aorta with dissection extending
down the entire aorta. Type II has an intimal tear in the ascending aorta with dissection
limited to the ascending aorta. Type III has an intimal tear in the proximal descending
thoracic aorta with dissection limited to the thoracic aorta (type IIIA) or extending
distally to the abdominal aorta or aortoilic bifurcation (type IIIB). (Adapted
from DeBakey ME, Beall AC Jr, Cooley DA, et al: Dissecting aneurysms of the aorta.
Surg Clin North Am 46:1045–1055, 1966.)
Figure 52-15
The DeBakey classification of dissecting aneurysms of
the aorta. Type I has an intimal tear in the ascending aorta with dissection extending
down the entire aorta. Type II has an intimal tear in the ascending aorta with dissection
limited to the ascending aorta. Type III has an intimal tear in the proximal descending
thoracic aorta with dissection limited to the thoracic aorta (type IIIA) or extending
distally to the abdominal aorta or aortoilic bifurcation (type IIIB). (Adapted
from DeBakey ME, Beall AC Jr, Cooley DA, et al: Dissecting aneurysms of the aorta.
Surg Clin North Am 46:1045–1055, 1966.)
inadequate response to medical therapy. Approximately 20% to 40% of patients with
chronic aortic dissection will develop significant aneurysmal dilatation of the descending
thoracic or thoracoabdominal aorta.[325]
 Figure 52-14
The Crawford classification of thoracoabdominal aortic
aneurysms is defined by anatomic location and the extent of involvement. Type I
aneurysms involve all or most of the descending thoracic aorta and the upper abdominal
aorta; type II aneurysms involve all or most of the descending thoracic aorta and
all or most of the abdominal aorta; type III aneurysms involve the lower portion
of the descending thoracic aorta and most of the abdominal aorta; and type IV aneurysms
involve all or most of the abdominal aorta, including the visceral segment. (Adapted
from Crawford ES: Thoracoabdominal and suprarenal abdominal aortic aneurysm. In
Ernst CB, Stanley JC [eds]: Current Therapy in Vascular Surgery. Philadelphia,
Decker, 1987, pp 96–98.)
Figure 52-14
The Crawford classification of thoracoabdominal aortic
aneurysms is defined by anatomic location and the extent of involvement. Type I
aneurysms involve all or most of the descending thoracic aorta and the upper abdominal
aorta; type II aneurysms involve all or most of the descending thoracic aorta and
all or most of the abdominal aorta; type III aneurysms involve the lower portion
of the descending thoracic aorta and most of the abdominal aorta; and type IV aneurysms
involve all or most of the abdominal aorta, including the visceral segment. (Adapted
from Crawford ES: Thoracoabdominal and suprarenal abdominal aortic aneurysm. In
Ernst CB, Stanley JC [eds]: Current Therapy in Vascular Surgery. Philadelphia,
Decker, 1987, pp 96–98.) Figure 52-15
The DeBakey classification of dissecting aneurysms of
the aorta. Type I has an intimal tear in the ascending aorta with dissection extending
down the entire aorta. Type II has an intimal tear in the ascending aorta with dissection
limited to the ascending aorta. Type III has an intimal tear in the proximal descending
thoracic aorta with dissection limited to the thoracic aorta (type IIIA) or extending
distally to the abdominal aorta or aortoilic bifurcation (type IIIB). (Adapted
from DeBakey ME, Beall AC Jr, Cooley DA, et al: Dissecting aneurysms of the aorta.
Surg Clin North Am 46:1045–1055, 1966.)
Figure 52-15
The DeBakey classification of dissecting aneurysms of
the aorta. Type I has an intimal tear in the ascending aorta with dissection extending
down the entire aorta. Type II has an intimal tear in the ascending aorta with dissection
limited to the ascending aorta. Type III has an intimal tear in the proximal descending
thoracic aorta with dissection limited to the thoracic aorta (type IIIA) or extending
distally to the abdominal aorta or aortoilic bifurcation (type IIIB). (Adapted
from DeBakey ME, Beall AC Jr, Cooley DA, et al: Dissecting aneurysms of the aorta.
Surg Clin North Am 46:1045–1055, 1966.)