|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The potential for significant and rapid blood loss cannot be underestimated. I most commonly use one 8.5F internal jugular catheter and one or two large-bore peripheral catheters. A hemostatic valve with a side port is placed on the 8.5F catheter to accept a central venous or a pulmonary artery catheter. Placement of arterial catheters should be routine for all patients undergoing abdominal aortic reconstruction. As with other vascular procedures, the radial artery is most commonly selected for cannulation because of its superficial location, easy accessibility, and low complication rate. A noninvasive blood pressure cuff should be placed on the arm contralateral to the arterial catheter in the event of catheter malfunction.
Placement of central venous catheters is routine for all open aortic procedures. Such catheters allow central venous pressure monitoring and administration of drugs into the central circulation. The routine, nonselective use of pulmonary artery catheter monitoring is not recommended for all patients undergoing infrarenal abdominal aortic reconstruction.[263] [264] For these procedures, pulmonary artery catheters should be considered for patients with
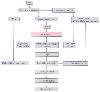 Figure 52-13
Systemic hemodynamic response to aortic unclamping.
AoX, aortic cross-clamping; Cven, venous capacitance; R art, arterial resistance;
Rpv, pulmonary vascular resistance. (From Gelman S: The pathophysiology
of aortic cross-clamping and unclamping. Anesthesiology 82:1026–1060, 1995.)
Figure 52-13
Systemic hemodynamic response to aortic unclamping.
AoX, aortic cross-clamping; Cven, venous capacitance; R art, arterial resistance;
Rpv, pulmonary vascular resistance. (From Gelman S: The pathophysiology
of aortic cross-clamping and unclamping. Anesthesiology 82:1026–1060, 1995.)
With selective use, accurate interpretation of data, and rational treatment strategies, pulmonary artery catheter monitoring can be beneficial in high-risk patients undergoing complex aortic reconstruction. However, the clinical value of pulmonary artery catheter monitoring in high-risk patients has not been established. [266] Clinical studies over the past 2 decades have yielded quite conflicting results. Some studies have reported a decrease in mortality, [67] some have observed no effect,[267] and others have reported an increase in mortality.[268] Consensus statements have been published,[269] [270] most recently by the National Heart, Lung and Blood Institute and the U.S. Food and Drug Administration (FDA).[270] A large prospective, randomized trial compared goal-directed therapy guided by a pulmonary artery catheter with standard care without the use of a pulmonary artery catheter in high-risk surgical patients and reported no benefit of treatment guided by a pulmonary artery catheter.[271] This study did not find an increase in mortality with insertion and use of a pulmonary artery catheter.
Two-dimensional TEE has been used intraoperatively to assess global ventricular function, guide fluid therapy, and monitor for myocardial ischemia. Continuous monitoring of ventricular function is commonly obtained from a single short-axis view at the level of the mid-papillary muscle. Using this same view, visualization of the left ventricle at end-diastole allows rapid assessment of ventricular filling (i.e., preload). Ejection fraction area can be calculated by use of left ventricular end-diastolic and end-systolic areas. In patients undergoing abdominal aortic reconstruction, echocardiographic short-axis left ventricular end-diastolic area, end-systolic area, and ejection fraction area correlate closely with changes in corresponding left ventricular volumes and ejection fraction obtained by radionuclide angiography.[272] [273] Patients requiring supraceliac aortic cross-clamping have significant increases in end-diastolic area and significant decreases in ejection fraction by echocardiography that are not completely normalized with vasodilators and frequently are not detected by pulmonary artery catheter monitoring.[216]
TEE can also reveal abnormalities of left ventricular wall motion and wall thickening. The relationship between these abnormalities and coronary perfusion has been established experimentally[274] [275] and clinically,[276] [277] and such abnormalities may precede electrocardiographic evidence of ischemia.[153] The short-axis view at the level of the midpapillary muscles allows assessment of all three major coronary distributions. TEE-detected segmental wall motion abnormalities may occur in up to 37% of patients during infrarenal aortic reconstruction[278] and in more than 90% of patients requiring supraceliac aortic cross-clamping.[216] [238] The natural history of echocardiographic segmental wall motion abnormalities was studied in 156 patients at risk for cardiac morbidity undergoing noncardiac surgery.[278] The 24 patients undergoing aortic surgery had the highest incidence of new segmental wall motion abnormalities (38%). Surprisingly, the incidence of new segmental wall motion abnormalities did not differ for patients with known CAD and those with cardiac risks factors only. Ischemic episodes detected by TEE correlated poorly with postoperative cardiac complications. The authors also reported a discordant relation between new segmental wall motion abnormalities and electrocardiographic ischemic changes. In a subsequent report, a subset of 285 patients who were at high risk for cardiac complications and were undergoing noncardiac surgery were studied with continuous intraoperative use of two-lead ECG, 12-lead ECG, and TEE for the detection of ischemia.[279] A lack of concordance was found for the three different ischemia monitors. When compared with preoperative clinical data and intraoperative monitoring in which two-lead ECG was used, routine monitoring for myocardial ischemia with TEE or 12-lead ECG during noncardiac surgery had little incremental clinical value in identifying patients at high risk for perioperative ischemic outcomes. Available data are insufficient to define the sensitivity and specificity of segmental wall motion abnormalities as a marker for myocardial ischemia or as a predictor of perioperative ischemic outcomes.
The optimal intraoperative monitoring techniques for patients undergoing abdominal aortic reconstruction have not been established. Existing clinical studies offer insufficient data to conclusively answer the question of whether pulmonary artery catheter or TEE monitoring improves outcome. The clinical usefulness of any monitoring technique ultimately depends on patient selection, accurate interpretation of data, and appropriate therapeutic intervention.
Elective abdominal aortic reconstruction may result in substantial blood loss and warrants the routine cross-matching of 4 to 6 units of packed red blood cells. Suprarenal aneurysms and other more complex aortic reconstructions often demand even greater allogeneic blood availability. Over the past 2 decades, concerns regarding the safety, availability, and acceptability of allogeneic blood have led to greater use of autologous blood procurement (see Chapter 47 and Chapter 48 ). Preoperative autologous donation,[280] [281] [282] intraoperative cell salvage,[283] [284] [285] and acute normovolemic hemodilution[286] [287] [288] have been used during aortic surgery to reduce or eliminate exposure to allogeneic blood and the associated risks of transfusion-related complications.
Intraoperative cell salvage is the most widely used technique, and in some centers, it is considered routine. The equipment is expensive and requires significant training and expertise. An early, nonrandomized study reported a 75% reduction in the number of allogeneic red blood cell units transfused during elective aortic surgery with the use of cell salvage.[283] Later randomized studies reported conflicting results.[284] [285] The routine use of cell salvage during aortic surgery may not be a cost-effective measure ($250 to $350 per case), and it may be best reserved for a select group of patients with high expected blood loss.[289] The equivalent of at least 2 units of washed blood needs to be recovered for this technique to be cost effective.[290] A cost-effective option is to use the cell salvage reservoir for blood collection and activate the full salvage process if large blood loss occurs.
Acute normovolemic hemodilution is often used in combination with intraoperative cell salvage during aortic surgery. Two randomized studies reported the combined use of hemodilution and cell salvage reduced the allogeneic blood requirement in patients undergoing aortic surgery.[286] [288] As with cell salvage alone, this benefit is likely greater with higher blood loss procedures. Another randomized study found that the combined use of the two techniques during aortic surgery was cost neutral compared with standard allogeneic transfusion. [291] Hemodilution does not worsen myocardial ischemia and may improve hemodynamic tolerance to aortic cross-clamping in patients with CAD. [292]
A variety of anesthetic techniques and drugs, including general anesthesia, regional (epidural) anesthesia, and combined techniques, have been used successfully for abdominal aortic reconstruction. Combined techniques most commonly employ a lumbar or low thoracic epidural catheter in addition to a "light" general anesthetic. Local anesthetics, opioids, or more commonly, a combination of the two, may be administered by bolus or continuous epidural infusion. Evidence suggests that maintenance of vital organ perfusion and function by the provision of stable perioperative hemodynamics is more important to overall outcome than the choice of anesthetic agent or technique. The specific anesthetic technique for patients undergoing abdominal aortic reconstruction is important insofar as it allows rapid and precise control of hemodynamic parameters. Given the high incidence of cardiac morbidity and mortality in patients undergoing aortic reconstruction, special emphasis must be placed on factors that influence ventricular work and myocardial perfusion. In general, a balanced anesthetic using relatively short-acting agents maximizes management flexibility, which is most desirable in this patient population.
Induction of general anesthesia should proceed in a controlled fashion such that a stable hemodynamic profile is maintained during loss of consciousness, laryngoscopy and intubation, and the immediate postinduction period. A variety of intravenous hypnotic agents (thiopental, etomidate, propofol) are suitable. The addition of a short-acting, potent opioid, such as fentanyl
Anesthetic maintenance may be accomplished with a combination of a potent opioid (fentanyl or sufentanil) and an inhaled anesthetic (sevoflurane, desflurane, or isoflurane). Patients with severe left ventricular dysfunction may benefit from a pure opioid technique, but a balanced anesthetic technique allows the clinician to take advantage of the most desirable characteristics of potent opioids and inhalational agents while minimizing their undesirable side effects. I typically use low-dose isoflurane in 50% nitrous oxide and 15 to 20 µg/kg of fentanyl. Approximately 50% of the opioid dose is administered during induction and before skin incision. When epidural local anesthetics are used, I employ the same balanced technique and reduce the fentanyl dose to 8 to 10 µg/kg.
Nitrous oxide can be used to supplement an opioid or an inhalational-based anesthetic. In general, nitrous oxide has a tendency to decrease cardiac output and arterial pressure while increasing systemic vascular resistance. One report indicated that in patients undergoing abdominal aortic surgery, nitrous oxide increased the need for vasodilator therapy to treat increases in pulmonary capillary wedge pressure and myocardial ischemia.[293] I typically use 50% nitrous oxide as part of a balanced anesthetic technique, particularly when extubation is planned at the termination of the procedure.
Various regional anesthetic and analgesic techniques have been used effectively during and after aortic reconstruction. An overview of the advantages and limitations of regional techniques for vascular surgery is included in the discussion of lower extremity vascular surgery, and the discussion here is limited to the clinical issues specific to abdominal aortic reconstruction. For more than a decade, considerable interest has been focused on the use of regional anesthetic and analgesic techniques to reduce the incidence of perioperative morbidity in patients undergoing aortic reconstruction. The benefits of combined general-epidural anesthesia intraoperatively, with or without epidural analgesia continued into the postoperative period, remain controversial, and conflicting results have been reported.[132] [294] [295] [296] [297] [298] Moreover, studies that have reported improved outcome do not determine whether the benefit results from the intraoperative anesthetic technique or from the postoperative pain regimen (or from a combination of the two). In a randomized trial using epidural morphine in patients undergoing aortic surgery, Breslow and associates[299] found an attenuation of the adrenergic response and a lower incidence of hypertension in the postoperative period. A large randomized trial reported no reduction in nonsurgical complications with the use of intrathecal opioid.[300] The effects of anesthetic or analgesic technique on the incidence of perioperative myocardial ischemia have received considerable attention. Four randomized trials, with nearly 450 combined patients undergoing aortic reconstruction, failed to demonstrate a reduction in the incidence of perioperative,[132] [301] intraoperative,[239] or postoperative[297] myocardial ischemia when epidural techniques were employed. Randomized trials have not demonstrated a reduction in the incidence of cardiovascular, pulmonary, or renal complications after aortic surgery with the use of epidural techniques.[132] [295] [296] [297] [302]
The duration and intensity of postoperative care after aortic surgery critically depend on the physiologic derangements incurred during the perioperative period (i.e., depression of consciousness, hypothermia, fluid overload, incisional pain, ileus, and respiratory depression) and the development of certain less common, but more severe, postoperative complications (i.e., MI, pneumonia, sepsis, renal failure, and decreased tissue perfusion). Length of hospital stay may therefore be considered the outcome variable most directly proportional to an integrated final negative effect of all significant perioperative morbidity (excluding in-hospital death) and the variable most likely to be altered by anesthetic or analgesic technique. Randomized trials have not demonstrated any reduction in length of hospital stay after aortic surgery with the use of regional techniques.[132] [295] [296] [297] [300] [302] Results were reported for a randomized clinical trial comparing alternate combinations of intraoperative anesthesia (i.e., general and combined epidural-general) and postoperative analgesia (i.e., intravenous patient-controlled analgesia and epidural patient-controlled analgesia) with respect to length of stay after abdominal aortic surgery.[132] Two unique features of the trial included a factorial design, which allowed for the inclusion of all four combinations of intraoperative anesthesia and postoperative analgesia and the ability to separate the influence of time period and technique, and a double-blind design, which helped to eliminate bias. The study rigorously protocolized perioperative management, standardized postoperative surgical care, and optimized postoperative pain management. Although the overall length of stay (median, 7.0 days) was much lower than that reported in other studies,[295] [296] [297] [302] my colleagues and I were not able to demonstrate a reduction in length of stay based on anesthetic or analgesic technique. The overall incidence of postoperative complications in the trial was low and not different based on anesthetic or analgesic technique. Postoperative pain was well controlled overall, with similar pain scores in both analgesic treatment groups. It is my conclusion based on these results that if perioperative care and pain relief are optimized (and adverse outcomes minimized), epidural anesthetic and analgesic techniques for aortic surgery offer no major advantage or disadvantage compared with general anesthesia and intravenous patient-controlled analgesia. I recommend that anesthetic and analgesic selection for abdominal aortic surgery should be based primarily on the preferences of the physician and patient.
Clinical studies have identified several disadvantages in the use of epidural local anesthetics in combination with general anesthesia during aortic reconstruction, including significant hypotension at the time of aortic
I begin preparation for emergence from anesthesia after restoration of circulation and establishment of adequate organ perfusion. Hemodynamic, metabolic, and temperature homeostasis must be achieved before skin closure; otherwise, patients are transported to the intensive care unit intubated, with their ventilation controlled. Extubation of the trachea is generally not attempted in patients with supraceliac aortic cross-clamp times greater than 30 minutes, patients with poor baseline pulmonary function, or patients requiring large volumes of blood or crystalloid during surgery. At the start of skin closure, inhaled anesthetics are discontinued, nitrous oxide is increased to 70%, and residual neuromuscular blockade is reversed. I routinely place a large nasal airway after induction but before systemic heparinization in all patients for whom extubation is planned in the operating room. Hypertension and tachycardia are aggressively controlled during emergence by use of short-acting agents, such as esmolol, nitroglycerin, and sodium nitroprusside. Patients are placed in a recumbent, head-up position, and nitrous oxide is discontinued. If spontaneous ventilation is adequate, the trachea is extubated. Some centers advocate extubation of all patients in the intensive care unit after a period of stability has been established. In these cases, mild sedation with a benzodiazepine such as midazolam is appropriate.
Postoperative hypothermia is associated with many undesirable physiologic effects[306] [307] [308] and may contribute to adverse outcome[309] [310] [311] [312] (see Chapter 40 ). I maintain normothermia before skin incision by increasing ambient temperature in the operating room, applying warm cotton blankets, and warming the intravenous fluids. If significant hypothermia occurs early in the procedure, normothermia is extremely difficult to achieve, and emergence and extubation may be delayed. During surgery, all fluids and blood products should be warmed before administration. I routinely apply a forced-air warming blanket over the upper body. The lower body should not be warmed, because doing so can increase injury to ischemic tissue distal to the cross-clamp by increasing metabolic demands.
|
|
|
|
|
|
|
|
|
|
|
|
|