|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Assessing cardiac risk in patients before vascular surgery is a controversial and difficult task (see Chapter 24 ). In the mid-1970s, Goldman and colleagues[34] pioneered the concept of a "risk index" to account for the multifactorial nature of contributors to risk for cardiac morbidity. In this landmark study, 1000 patients scheduled for major surgical procedures were prospectively enrolled, and a multivariate model was used to determine the independent risk contribution of each of several clinical variables. Independent predictors of morbidity were identified as age older than 70 years; MI in the previous 6 months; an S3 gallop or jugular venous distention; any preoperative cardiac rhythm other than sinus; aortic stenosis; general medical problems (e.g., abnormal arterial blood gases, electrolytes, creatinine); and emergency, intrathoracic, or intra-abdominal surgery. Limitations of the study by Goldman and coworkers[34] include a relatively low-risk population (only 8% were vascular surgery patients), which
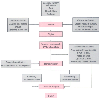 Figure 52-2
Algorithm of the Coronary Artery Revascularization Prophylaxis
Trial. AAA, abdominal aortic aneurysm; LV, left ventricular; LVEF, left ventricular
ejection fraction. (From Krupski WC: Update on perioperative evaluation
and management of cardiac disease in vascular surgery patients. J Vasc Surg 36:1292–1308,
2002.)
Figure 52-2
Algorithm of the Coronary Artery Revascularization Prophylaxis
Trial. AAA, abdominal aortic aneurysm; LV, left ventricular; LVEF, left ventricular
ejection fraction. (From Krupski WC: Update on perioperative evaluation
and management of cardiac disease in vascular surgery patients. J Vasc Surg 36:1292–1308,
2002.)
Since the publication of the risk index by Goldman and colleagues, [34] several other investigators have published risk indices in an attempt to increase the sensitivity and specificity for predicting perioperative morbidity.[35] [36] [37] [38] [39] [40] The patients in these series were also relatively low risk because only a small percentage underwent vascular surgery. Although risk indices are a cost-effective screening method for determining which patients require further cardiac evaluation, the high pretest probability of CAD in vascular surgery patients-makes the risk index less useful.
The history and physical examination are not very reliable for detecting CAD in the general population. In patients with peripheral vascular disease and significant claudication, the history and physical, particularly with regard to angina and exercise tolerance, may be even less reliable. The symptoms of ischemic heart disease are often not manifested in diabetic patients, and silent myocardial ischemia (by definition) goes unrecognized.[41] Despite these limitations, several important risk predictors can be obtained from the history and physical examination ( Table 52-2 ). These risk predictors are an integral part of the stepwise approach (see Fig. 52-3A and Fig. 52-3B ) to preoperative assessment recommended by the ACC/AHA.[32] History of a previous MI or congestive heart failure has been shown in multiple studies to predict risk for perioperative cardiac morbidity.[34] [42] [43] [44] Simple clinical markers (e.g., age older than 70 years, diabetes, angina, congestive heart failure, prior MI, prior CABG), weighted according to prognostic impact, have been shown to reliably stratify cardiac risk in vascular surgery patients referred for preoperative testing.[45]
Exercise tolerance, or functional capacity, is valuable information that may eliminate the need for preoperative cardiac testing.[46] For example, patients with adequate lower extremity blood flow (e.g., patients with isolated carotid disease) may have an exercise tolerance that indicates good left ventricular function and low likelihood of significant CAD. Perioperative risk is increased in patients with poor exercise tolerance.[47] Functional capacity, expressed by metabolic equivalents (METs), can be used to guide cardiac evaluation after risk stratification using clinical predictors.[32] [48]
It is not clear whether any specific category of vascular disease is associated with a greater likelihood of coexisting CAD. Some investigators have shown a similar incidence and severity of CAD in patients with aortic, lower extremity, and carotid disease.[49] Others have shown that patients with lower extremity vascular disease are more likely to have significant CAD and to experience perioperative morbidity.[50] [51] [52] In the ACC/AHA guidelines,[32] aortic and lower extremity procedures are considered high risk and carotid procedures intermediate risk for perioperative cardiac morbidity ( Table 52-3 ). The difference in these subgroups of vascular patients is related not to the incidence or the severity of coexisting disease, but rather to the invasiveness of the surgery.
Although hypertension is well recognized as a risk factor for atherosclerotic disease, multiple studies have shown that moderate hypertension is not an independent risk factor for perioperative cardiac morbidity.[36] [53] [54] Hypertensive patients may be more hemodynamically labile intraoperatively and postoperatively. [55] Patients often have acutely elevated blood pressures on admission to the hospital, especially the morning of surgery, because of anxiety This should be taken into account before patients are diagnosed with or treated for hypertension.[56] I recommend that several preoperative blood pressure measurements (e.g., clinic visit, preoperative visit, admission to hospital) be taken into consideration when determining a patient's "baseline" blood pressure.
Patients should be maintained with their usual antihypertensive medications throughout the perioperative period, with an oral dose given the morning of surgery and parenteral doses given thereafter in patients unable to take oral medications. Care should be taken to prevent withdrawal from β-blockers and clonidine to avoid a rebound increase in heart rate and blood pressure. The perioperative use of β-blockers and the control of heart rate have been shown to reduce the incidence of perioperative myocardial ischemia and subsequent cardiac morbidity.[26] [57] [58] [59] [60] [61] [62] [63] I view this as one of the most important advances in the perioperative care of vascular surgical patients. Clonidine decreases anesthetic requirements, catecholamine levels, and blood pressure lability.[64] [65] The effect of calcium channel blockers on perioperative cardiac morbidity has not been studied in a randomized fashion, but there is evidence that the incidence of myocardial ischemia is unaffected.[57] Because angiotensin-converting enzyme inhibitors may lead to intraoperative hypotension, it has been recommended that these drugs be avoided in the immediate preoperative period.[66] If necessary, blood pressure can be controlled with shorter-acting acute therapy during the intraoperative and early postoperative periods.
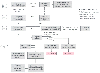 Figure 52-3a
Algorithm recommended by the American College of Cardiology/American
Heart Association Task Force on Practice Guidelines for a stepwise approach to preoperative
assessment. Subsequent care may include cancellation or delay of surgery, coronary
revascularization followed by noncardiac surgery, or intensified care. (Adapted
from Eagle KA, Berger PB, Calkins H, et al: ACC/AHA Guideline Update for Perioperative
Cardiovascular Evaluation for Noncardiac Surgery—Executive Summary: A report
of the American College of Cardiology/American Heart Association Task Force on Practice
Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular
Evaluation for Noncardiac Surgery). J Am Coll Cardiol 39:542–553, 2002.)
Figure 52-3a
Algorithm recommended by the American College of Cardiology/American
Heart Association Task Force on Practice Guidelines for a stepwise approach to preoperative
assessment. Subsequent care may include cancellation or delay of surgery, coronary
revascularization followed by noncardiac surgery, or intensified care. (Adapted
from Eagle KA, Berger PB, Calkins H, et al: ACC/AHA Guideline Update for Perioperative
Cardiovascular Evaluation for Noncardiac Surgery—Executive Summary: A report
of the American College of Cardiology/American Heart Association Task Force on Practice
Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular
Evaluation for Noncardiac Surgery). J Am Coll Cardiol 39:542–553, 2002.)
| Major |
| Unstable coronary syndromes |
| Acute or recent myocardial infarction with evidence of important ischemic risk by clinical symptoms or invasive testing |
| Unstable or severe angina (Canadian class III or IV) |
| Decompensated heart failure |
| Significant arrhythmias |
| High-grade atrioventricular block |
| Symptomatic ventricular arrhythmias in the presence of underlying heart disease |
| Supraventricular arrhythmias with uncontrolled ventricular rate |
| Severe valvular disease |
| Intermediate |
| Mild angina pectoris (Canadian class I or II) |
| Previous myocardial infarction by history or pathologic Q waves |
| Compensated or prior heart failure |
| Diabetes mellitus |
| Renal insufficiency |
| Minor |
| Advanced age |
| Abnormal electrocardiogram |
| Rhythm other than sinus |
| Low functional capacity |
| History of stroke |
| Uncontrolled systemic hypertension |
| Adapted from Eagle KA, Berger PB, Calkins H, et al: ACC/AHA Guideline Update for Perioperative Cardiovascular Evaluation for Noncardiac Surgery—Executive Summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). J Am Coll Cardiol 39:542–553, 2002. |
Patients who have had prior MIs are at greater risk for reinfarction
in the perioperative period than others ( Table
52-4
). Studies from the 1970s reported prevalence of perioperative MI
of about 30%, 15%, and 5% in patients who had a prior MI less than 3 months, 3 to
6 months, and more than 6 months before the procedure, respectively.[34]
[42]
[43]
[44]
In the 1980s, Rao and coworkers[67]
demonstrated
that risk for perioperative reinfarction could be reduced with careful intraoperative
and postoperative care that included invasive hemodynamic monitoring with arterial
and pulmonary artery catheters, control of hemodynamic parameters, and intensive
care after surgery. With this "extra care," the risk of reinfarction was reduced
to 6%, 2.5%, and 1.5% in patients who had had a prior MI less than 3 months, 3 to
6 months, and more than 6 months before the procedure, respectively.[67]
Of note, this study had significant limitations, including the retrospective design,
the lack of careful outcome surveillance, and the unidentified changes in
| High (reported cardiac risk often greater than 5%) |
| Emergent major operations, particularly in the elderly |
| Aortic and other major vascular surgery |
| Peripheral vascular surgery |
| Anticipated prolonged surgical procedures associated with large fluid shifts and blood loss |
| Intermediate (reported cardiac risk generally less than 5%) |
| Carotid endarterectomy |
| Head and neck surgery |
| Intraperitoneal and intrathoracic surgery |
| Orthopedic surgery |
| Prostate surgery |
| Low (reported cardiac risk generally less than 1%) |
| Endoscopic procedures |
| Superficial procedures |
| Cataract surgery |
| Breast surgery |
| From Eagle KA, Brundage BH, Chaitman BR, et al: Guidelines or perioperative cardiovascular evaluation for noncardiac surgery. Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). J Am Coll Cardiol 27:910–948, 1996, and from Eagle KA, Berger PB, Calkins H, et al: ACC/AHA Guideline Update for Perioperative Cardiovascular Evaluation for Noncardiac Surgery—Executive Summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). J Am Coll Cardiol 39:542–553, 2002. |
Given the increased risk for morbidity in patients with prior MI, traditional anesthetic practice dictated postponing elective surgery until a 6-month interval passed. It now appears that much of the risk after a prior MI is related to the functional status of the ventricles and the presence of ongoing ischemia, rather than to the actual age of the infarction.[32] [68] Although prospective data are not available, current guidelines recommend waiting 6 weeks after an uncomplicated MI before proceeding with an elective surgical procedure.[32] Vascular surgery, however, is often not elective. Urgent surgery is often necessary for patients with large or symptomatic aneurysms, transient ischemic attacks from carotid stenosis, or limb-threatening ischemia from iliac or femoral disease.
|
|
|
Rao et al.[67] |
|
|
|---|---|---|---|---|
| Time Elapsed between Prior Myocardial Infarction and Operation (months) | Tarhan et al.[44] (1972) | Before 1977 | 1977 and After | Shah et al.[723] (1990) |
| 0–3 | 37 | 36 | 5.8 | 4.3 |
| 4–6 | 16 | 26 | 2.3 | 0 |
| >6 | 5.6 | 5 | 1.5 | 5.7 |
| Time unknown | — | — | — | 3.3 |
A preoperative electrocardiogram (ECG) should be obtained for all patients undergoing vascular surgery. This ECG is necessary for comparison if myocardial ischemia or infarction is suspected postoperatively. If Q waves or other evidence of prior MI is present, then previous records should be reviewed to determine the timing of the infarct for the purpose of risk stratification. Dysrhythmias should be evaluated preoperatively to optimize perioperative management (e.g., rate control for patients in atrial fibrillation). The presence of a cardiac rhythm other than sinus indicates risk for perioperative cardiac morbidity,[34] [38] although it is unclear whether treatment of dysrhythmias reduces risk. Approximately 50% of patients with CAD have a normal resting ECG, demonstrating that the ECG lacks sensitivity for predicting cardiac morbidity.[69]
Exercise stress testing is a widely available and cost-effective method of screening for CAD in the general population. However, a substantial proportion of vascular surgery patients (30% to 70%) cannot attain target heart rates that allow detection of ischemia.[70] The inability to reach target heart rates is often caused by poor functional capacity or β-blocker therapy. Many elderly patients have knee and hip disorders, chronic lung disease, or previous stroke that limits their exercise capacity. One alternative is arm exercise, [71] but fatigue often precedes the increase in heart rate, and there has been little enthusiasm for this technique. If vascular surgery patients are able to exercise and achieve 85% of their predicted maximal heart rate, they are at substantially lower risk for perioperative cardiac morbidity.[72] One study demonstrated a fivefold increase in cardiac risk for patients who were unable to attain the target heart rate with significant ST changes.[72] However, larger studies do not support these findings, showing poor predictive values for exercise stress testing.[73] [74] When reviewing the results of an exercise stress test, useful information in addition to electrocardiographic changes includes the patient's symptoms, exercise capacity, and hemodynamic changes with exercise.
Dipyridamole thallium imaging (DTI) is the most commonly used noninvasive test to screen vascular surgery patients for CAD and risk for perioperative cardiac morbidity. Thallium 201, a radioactive potassium analog, is used to assess myocardial perfusion and viability. The initial distribution of thallium after intravenous injection is proportional to myocardial blood flow. Areas of myocardium that are hypoperfused show up on scintigraphic imaging as a relative "cold spot." Redistribution imaging, which is obtained 3 to 6 hours later, reflects myocardial viability and is unrelated to blood flow. Dipyridamole vasodilates normal coronary arteries by blocking reuptake of adenosine, but stenotic vessels have a fixed diameter and cannot dilate. This creates a steal phenomenon, whereby blood flow is redirected away from diseased coronary vascular beds. Thallium imaging is usually performed to detect hypoperfused areas of myocardium soon after the dipyridamole is given. A second image is obtained 3 to 6 hours later after the vasodilator effect has dissipated (redistribution phase) to determine myocardial viability. There are three general outcome results from this test. The first is a normal test result with complete perfusion on the first and second images. The second is one that shows myocardium at risk ("redistribution"), with the first image showing a hypoperfusion defect and the second image showing normal perfusion. This indicates coronary narrowing and ischemia that is induced by the vasodilator. The anatomic location of the area at risk can usually be assessed on the images. The third is a fixed perfusion defect indicating an old infarction that has scarred the myocardium to such a degree that blood flow is absent on both images.
There is ongoing controversy over the value of DTI for risk stratification in patients scheduled for vascular surgery. Two large studies in vascular surgery patients showed low sensitivity for DTI's predicting cardiac morbidity.[75] [76] This may be related to the fact that the population in these large studies had a lower risk than that of populations in other studies, in which selected patients were enrolled who had clinical risk factors (i.e., a higher pretest probability of morbidity). Table 52-5 illustrates the predictive value for DTI in vascular surgery patients.
The most rational use of DTI was proposed by Eagle and colleagues [77] in 1989. In this study, vascular surgery patients were stratified by clinical predictors and then by thallium redistribution ( Fig. 52-4 ). The clinical predictors
|
|
|
|
Perioperative Events No. (%) |
|
|
|---|---|---|---|---|---|
| Study | No. of Patients Who Underwent Surgery | Events (MI or Death) No. (%) | Predictive Value (Positive Test) | Predictive Value (Negative Test) | Comments |
| Dipyridamole Thallium Imaging * | |||||
| Boucher et al.[724] | 48 | 3 (6) | 3/16 (19) | 32/32 (100) | First to define risk using DTI |
| Leppo et al.[725] | 89 | 15 (16) | 14/42 (33) | 46/47 (98) |
|
| Eagle et al.[77] | 200 | 15 (8) | 13/82 (16) | 61/62 (98) | Combined clinical variables with DTI |
| Mangano et al.[76] | 60 | 3 (5) | 1/22 (5) | 19/20 (95) | Managing physicians blinded to DTI results |
| Baron et al.[75] | 457 | 22 (5) | 7/160 (4) | 195/203 (96) | Did not analyze for cardiac death (nonfatal MI only) |
| Bry et al.[28] | 237 | 17 (7) | 12/110 (11) | 97/97 (100) | Cost-effectiveness data included |
| Dobutamine Stress Echocardiography | |||||
| Lalka et al.[93] | 60 | 9 (15) | 7/30 (23) | 28/30 (93) | Aortic surgery (multivariate analysis) |
| Langan et al.[94] | 74 | 3 (4) | 3/18 (17) | 56/56 (100) | Aortic surgery |
| Eichelberger et al.[97] | 75 | 2 (3) | 2/27 (7) | 48/48 (100) | Managing physicians blinded to DSE results |
| Poldermans et al.[95] | 131 | 5 (4) | 5/35 (14) | 96/96 (100) | Managing physicians blinded to DSE results (multivariate analysis) |
| Davila-Roman et al.[96] | 88 | 2 (2) | 2/20 (10) | 68/68 (100) | Included long-term follow-up |
| Ambulatory Electrocardiography | |||||
| Raby et al.[80] | 176 | 4 (2) | 3/32 (10) | 143/144 (99) | 24–48 hr during ambulation |
| Pasternack et al.[86] | 200 | 9 (5) | 7/78 (9) | 120/122 (98) | Multivariate analysis |
| Mangano et al.[87] | 474 | 6 (4) | 1/26 (4) | 113/118 (96) | AECG immediately before surgery |
| Fleisher et al.[130] | 67 | 4 (6) | 2/16 (13) | 49/51 (96) | AECG immediately before surgery |
| Fleisher et al.[78] | 86 | 4 (5) | 2/20 (10) | 64/66 (97) | Quantitative monitoring not predictive |
| AECG, ambulatory electrocardiography; DSE, dobutamine stress echocardiography; DTI, dipyridamole thallium imaging; MI, myocardial infarction. | |||||
| Adapted from Eagle KA, Brundage BH, Chaitman BR, et al: Guidelines for perioperative cardiovascular evaluation for noncardiac surgery. Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). J Am Coll Cardiol 27:910–948, 1996. | |||||
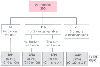 Figure 52-4
Algorithm for combining clinical variables and dipyridamole-thallium
results to stratify cardiac risk in vascular surgery patients. Cardiac events include
unstable angina, myocardial infarction, ischemic pulmonary edema, and cardiac death.
Clinical variables are the Q wave on electrocardiogram, age older than 70 years,
history of angina, ventricular ectopic activity requiring treatment, and diabetes
mellitus requiring treatment. (From Eagle KA, Coley CM, Newell JB, et al:
Combining clinical and thallium data optimizes preoperative assessment of cardiac
risk before major vascular surgery. Ann Intern Med 110:859–866, 1989.)
Figure 52-4
Algorithm for combining clinical variables and dipyridamole-thallium
results to stratify cardiac risk in vascular surgery patients. Cardiac events include
unstable angina, myocardial infarction, ischemic pulmonary edema, and cardiac death.
Clinical variables are the Q wave on electrocardiogram, age older than 70 years,
history of angina, ventricular ectopic activity requiring treatment, and diabetes
mellitus requiring treatment. (From Eagle KA, Coley CM, Newell JB, et al:
Combining clinical and thallium data optimizes preoperative assessment of cardiac
risk before major vascular surgery. Ann Intern Med 110:859–866, 1989.)
L'Italien and coworkers[45] expanded on Eagle's model for perioperative risk assessment using a large, diverse cohort of 1081 vascular surgical patients. DTI provided no additional stratification for patients classified as low or high risk according to simple clinical variables weighted according to prognostic impact. However, as with Eagle's earlier model, DTI reclassified more than 80% of the intermediate-risk (according to clinical variables) patients into low- and high-risk categories. The researchers suggested that DTI testing could be eliminated for the nearly 50% of patient classified as low or high risk based on clinical variables. Despite ongoing debate, DTI continues to be widely used for risk stratification in patients scheduled for vascular surgery.
Ambulatory electrocardiographic (AECG) monitoring has been used for many years to detect dysrhythmias, but only since the late 1980s has ambulatory monitoring for myocardial ischemia been used to predict risk for perioperative cardiac morbidity. As with DTI, the sensitivity of AECG monitoring for predicting cardiac morbidity is greatest in patients with high pretest probability. Preoperative ST segment changes consistent with myocardial ischemia occur in 20 to 40% of vascular surgery patients, and 80% to 90% of these ischemic episodes are silent.[78] [79] [80] The advantage of AECG monitoring over DTI is that AECG is approximately one third of the cost of DTI.[80] A disadvantage is the inability to test patients with significant electrocardiographic abnormalities that preclude ECG monitoring for ischemia (i.e., left bundle branch block, pacemaker dependency, and left ventricular hypertrophy with significant strain or digitalis effect).[81] Silent myocardial ischemia is known to predict adverse outcomes as a preoperative test and in other clinical settings. Silent ischemia predicts morbidity after MI [82] and in unstable angina.[83] Postoperative silent ischemia is also predictive of cardiac morbidity[84] and mortality[85] after peripheral vascular surgery.
Studies by Pasternack and associates[86] and Raby and colleagues[80] that included only vascular surgery patients found AECG monitoring to be highly sensitive (0.80 to 0.90) and specific (0.70 to 0.90). Other studies, including the largest series (474 patients) by Mangano and coworkers,[87] included general surgery patients, with a resulting lower sensitivity (0.30 to 0.50) and a similar specificity (0.70 to 0.80). Fleisher and associates[78] found that preoperative AECG monitoring had predictive value similar to that of DTI, although the two tests together provided more predictive ability than either test alone. Two disadvantages of AECG testing were reported. The highest-risk patients could not be monitored because of baseline ECG abnormalities, and cardiac risk was not quantifiable by the severity of ischemia on AECG but was quantifiable by DTI. Table 52-5 compares the results of several studies on AECG monitoring for risk stratification before vascular surgery.
Two-dimensional echocardiography is a widely available, noninvasive, relatively low capital cost technique that provides measurements of left ventricular function, ejection fraction, regional wall motion, and valvular function. Although the left ventricular function measured by echocardiography is a more qualitative measure than a quantitative one, this method is generally less expensive than radionuclide ventriculography, which is more quantitative.[88] Echocardiography has limited ability to measure ejection fraction. These measurements depend on loading conditions (i.e., preload and afterload).[89] Measurements of ventricular function are often made in the acute post-MI setting, resulting in patients being labeled with a poor ejection fraction long after the myocardium has recovered from the ischemic insult. Although studies have correlated left ventricular function with outcome in patients with acute MI,[90] the value of echocardiographic assessment as a predictor of perioperative cardiac morbidity has not been defined.
A study of 1351 consecutive vascular surgical patients suggests that the echocardiogram at rest may provide some prognostic information.[91] Patients with five or more abnormal segments had a fourfold to sixfold increased risk of cardiac complications compared with those with 4 or less abnormal segments.
Echocardiography can be used with pharmacologic stress testing (dobutamine or dipyridamole) to provoke myocardial ischemia, as detected by new or worsening wall motion abnormalities. Although not widely used, transesophageal imaging may be superior to transthoracic imaging for identifying the presence and extent of CAD, specifically in patients with poor acoustic windows.[92]
A meta-analysis found DSE to be the best predictor of cardiac morbidity (relative risk [RR] = 6.2), followed by DTI (RR = 4.6), radionuclide ventriculography (RR = 3.7), and AECG (RR = 2.7).[99] Table 52-5 compares the results for several studies on DSE for risk stratification before vascular surgery. Advances in echocardiography, including contrast assessment of myocardial perfusion and real-time three-dimensional imaging, may improve sensitivity and expand its usefulness in the preoperative assessment of vascular surgery patients.
Radionuclide ventriculography provides an accurate assessment of left ventricular function during rest or with exercise. The test is performed by quantitative analysis of sequential count densities of gated blood-pool images. In patients undergoing lower extremity arterial bypass or abdominal aortic resection, radionuclide ventriculography has been shown to be an independent predictor of perioperative cardiac morbidity. Pasternack and associates[100] [101] found that an ejection fraction of less than 35% was associated with a 75% to 85% rate of perioperative MI, and an ejection fraction of greater than 35% was associated with a 19% to 20% rate. Other investigators, however, have not been able to reproduce these results by use of radionuclide ventriculography at rest or with exercise.[102] [103] Although measurements of left ventricular function may play a role in defining a patient's long-term prognosis, the value of routinely measuring ejection fraction preoperatively is limited and its use in the vascular surgical population is declining. [104]
In summary, all three of the most commonly used preoperative ischemia tests (i.e., DTI, AECG, and DSE) have relatively high negative predictive values. If a patient tests negative (i.e., no ischemia detected) on these screening tests, it is unlikely that a morbid cardiac event will occur. However, low risk does not equal zero risk, and a negative test result should never be considered a guarantee that the patient does not have CAD. None of the ischemia tests discussed has a high positive predictive value, indicating that many patients with positive test results (i.e., ischemia detected) will not have a morbid cardiac event. Accurate clinical assessment of pretest probability of significant CAD is necessary for prudent use and rational interpretation of preoperative testing. Preoperative testing should not be undertaken if it is unlikely to alter the patient's management. Testing should not be considered as a preliminary step leading to coronary revascularization, because it is rarely necessary to perform a revascularization procedure solely for the purpose a getting a patient through the perioperative period. Further risk assessment of select patients may require invasive testing.
The largest series on outcome in vascular surgery patients is that of Hertzer and colleagues[49] from the Cleveland Clinic. These investigators performed cardiac catheterization in 1000 consecutive patients presenting for peripheral vascular surgery (aortic, carotid, or lower extremity revascularization). The incidence and severity of CAD was assessed according to the following classification: normal coronary arteries; mild-to-moderate CAD with no lesion exceeding 70% stenosis; advanced, compensated CAD with one or more lesions exceeding 70% stenosis but with adequate collateral circulation; severe, correctable CAD with greater than 70% stenosis in one or more coronary arteries; and severe inoperable CAD with greater than 70% stenosis in one or more coronary arteries with severe distal disease or poor ventricular function. The most remarkable findings were that only 8.5% of patients had normal coronary arteries and that 60% of patients had advanced or severe coronary lesions (>70% stenosis).[49] Even when CAD was not suspected by clinical history, more than one third of patients had advanced or severe coronary lesions. The results are shown in Table 52-6 .
In the series of Hertzer and colleagues,[105] the patients with severe correctable CAD were offered coronary bypass surgery, and if they consented, this surgery was performed before their peripheral vascular procedure. Patients who had normal or mild-to-moderate CAD went on to undergo vascular surgery, and those with severe inoperable disease were treated on an individual basis. Combined mortality rates over the immediate and long-term (4.6-year follow-up) postoperative periods are shown in Table 52-7 . Of the 216 patients who underwent coronary revascularization (CABG), 12 (5.5%) died after this surgery. This mortality rate is greater than the mortality rate reported for patients undergoing CABG surgery without peripheral vascular disease (1% to 2%), which suggests that the risks of CABG should be seriously considered as part of the preoperative evaluation of these patients. When overall early and late mortality (>5 years) were considered in the series by Hertzer and coworkers, death occurred in 12% versus 26% of patients who did or did not undergo CABG, respectively.[105] Although these data appear to support the beneficial effect of CABG on outcome, the mortality from CABG itself (5.5%) reduces its apparent benefits. No large-scale randomized trial has been performed to determine the impact of routine coronary angiography (with subsequent PTCA or CABG) before vascular surgery. The Coronary Artery Revascularization Prophylaxis Trial will likely provide extremely useful information to guide preoperative decision-making. Practice guidelines for coronary angiography have been developed by the ACC/AHA Task Force on Practice Guidelines.[106]
Patients who have undergone prior coronary artery bypass who are without current symptoms of angina or heart failure appear to have a relatively low incidence of perioperative cardiac morbidity.[32] When 12 studies were
|
|
Clinical CAD | |||||
|---|---|---|---|---|---|---|
|
|
None | Suspected | Total | |||
| Angiographic Classification | No. | % | No. | % | No. | % |
| Normal coronary arteries | 64 | 14 | 21 | 4 | 85 | 8.5 |
| Mild to moderate CAD | 218 | 49 | 99 | 18 | 317 | 32 |
| Advanced compensated CAD | 97 | 22 | 192 | 34 | 289 | 29 |
| Severe, correctable CAD | 63 | 14 | 188 | 34 | 251 | 25 |
| Severe, inoperable CAD | 4 | 1 | 54 | 10 | 58 | 5.8 |
| CAD, coronary artery disease | ||||||
| Data from Hertzer NR, Beven EG, Young JR, et al: Coronary artery disease in peripheral vascular patients. A classification of 1000 coronary angiograms and results of surgical management. Ann Surg 199:223–233, 1984. | ||||||
An even more difficult question is the role of interventional cardiology in the preoperative management of the vascular surgery patient. Patients with peripheral vascular disease are often not ideal candidates for PTCA, because this procedure requires a large-diameter introducer sheath in the femoral artery, which predisposes to pseudo-aneurysm and compromised blood flow to the lower extremities. [110] Although PTCA can be performed through the brachial artery, it is technically difficult. No randomized trials have been performed to determine the effects of PTCA on subsequent risk for cardiac morbidity in patients undergoing noncardiac surgery. There is evidence from published series that the risk of perioperative cardiac morbidity is relatively low in high-risk patients treated with PTCA preoperatively. In a retrospective review of 148 patients undergoing noncardiac surgery an average of 90 days after PTCA, only 1 patient had an MI.[111] In another series with 50 patients whose noncardiac surgery was performed an average of 9 days after PTCA, there were three MIs and one death. These patients had class 3 and 4 angina before their PTCA.[112] Because many of these patients did not have peripheral vascular disease, PTCA may have been technically easier to perform than in vascular patients.
There is considerable uncertainty regarding the optimal interval between PTCA and subsequent noncardiac surgical procedures. Approximately 25% to 35% of patients may have restenosis within a 6-month period after PTCA. Use of coronary stents has improved the capability of interventional cardiology to treat and prevent restenosis. Antiproliferative drug-eluting stents have demonstrated dramatic reductions in the rate of restenosis.[113] Available data suggest that, whenever possible, noncardiac surgery should be delayed 6 weeks after stent placement.[114] This interval generally allows adequate time for endothelialization of the stent, and completion of a course of antiplatelet therapy to prevent stent thrombosis. Noncardiac surgery within 6 weeks of stent placement has been associated with extremely high morbidity (i.e., MI and major bleeding) and mortality.[115]
Respiratory complications are potentially serious in patients undergoing vascular procedures. Given the prevalence of cigarette smoking in this population, chronic obstructive pulmonary disease and chronic bronchitis are common. When clinical assessment suggests severe pulmonary compromise, pulmonary function tests may be useful in evaluating and optimizing respiratory function (see Chapter 26 ). Preoperative blood gas determination can be used to establish a baseline for postoperative comparison. Baseline hypercapnia (partial pressure of arterial carbon dioxide > 45 mm Hg) indicates a higher risk for postoperative morbidity. [116] Given proper pulmonary care, even patients with severe pulmonary insufficiency, however, may undergo surgery with acceptable morbidity and mortality. Preoperative treatment with a short course of glucocorticoids (prednisone, 40 mg/day for 2 days) is helpful for patients with significant chronic obstructive pulmonary disease or asthma.[117] Although there is limited evidence for improved pulmonary outcome with regional anesthesia, [118] patients with significant pulmonary disease may benefit from epidural anesthesia or analgesia that is maintained into the postoperative period because this technique helps avoid respiratory depression from systemic opiates.
|
|
|
Normal or Mild to Moderate CAD | Advanced Compensated CAD | Severe, Correctable CAD with Bypass | No Bypass | Severe Inoperable CAD | Total Cardiac Deaths | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical Features | Total No. of Patients | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % |
| Men | 685 | 10/242 | 4.1 | 33/204 | 16 | 13/174 | 7.5 | 6/24 | 25 | 14/41 | 34 | 76 | 11 |
| Women | 315 | 5/160 | 3.1 | 12/85 | 14 | 12/42 | 29 | 3/11 | 27 | 8/17 | 47 | 40 | 13 |
| Age < 70 yr | 722 | 10/328 | 3.0 | 29/198 | 15 | 19/148 | 13 | 3/20 | 15 | 13/28 | 46 | 74 | 10 |
| Age > 70 yr | 278 | 5/74 | 6.8 | 16/91 | 18 | 6/68 | 8.8 | 6/15 | 40 | 9/30 | 30 | 42 | 15 |
| Normotensive | 403 | 7/185 | 3.8 | 15/102 | 15 | 8/82 | 9.8 | 2/15 | 13 | 8/19 | 42 | 40 | 9.9 |
| Hypertensive | 597 | 8/217 | 3.4 | 30/187 | 16 | 17/134 | 13 | 7/20 | 35 | 14/39 | 36 | 76 | 13 |
| Nondiabetic | 830 | 12/348 | 3.4 | 28/232 | 12 | 17/183 | 9.3 | 8/30 | 27 | 13/37 | 35 | 78 | 9.4 |
| Diabetic | 170 | 3/54 | 5.5 | 17/57 | 30 | 8/33 | 24 | 1/5 | 20 | 9/21 | 43 | 38 | 22 |
| Total | 1000 | 15/402 | 3.7 | 45/289 | 16 | 25/216 | 12 | 9/35 | 26 | 22/58 | 38 | 116 | 12 |
| CAD, coronary artery disease. | |||||||||||||
| From Hertzer NR, Young JR, Beven EG, et al: Late results of coronary bypass in patients with peripheral vascular disease. II. Five-year survival according to sex, hypertension, and diabetes. Cleve Clin J Med 54:15–23, 1987. | |||||||||||||
Underlying renal disease is common in vascular surgery patients. Hypertension itself may cause renal insufficiency or failure. Atherosclerotic disease in the abdominal aorta or renal arteries may compromise renal blood flow and renal function. Conversely, renal artery stenosis causes hypertension through renin-induced and angiotensin-induced vasoconstriction. Diabetic nephropathy is also common. Superimposed on baseline abnormalities in renal function are the preoperative and intraoperative angiographic dye loads, which are directly nephrotoxic,[119] and the interruption in renal blood flow from aortic cross-clamping. Even with infrarenal aortic cross-clamps, there can be a significant reduction in renal blood flow despite a normal systemic arterial blood pressure and cardiac output.[120] Embolic plaque can be showered into the renal arteries, especially when suprarenal aortic cross-clamps are applied and released.[121] Fluctuations in intravascular volume and cardiac output can compromise renal perfusion during the intraoperative and postoperative periods. In one series of more than 500 patients, the prevalence of acute renal failure was 7% after abdominal aortic reconstruction.[122]
|
|
|
|
|
|
|
|
|
|
|
|
|