|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The pathobiology of CPB has been discussed earlier. In addition, specific aspects of CPB management, including temperature, blood pressure, flow, flow patterns, rheology, and acid-base management, can modulate organ function during CPB and influence complications postoperatively.
Absent deep hypothermic circulatory arrest, the vast majority of centers perform cardiac surgery with CPB by using hypothermia at temperatures ranging from 27°C to 32°C. The use of hypothermia is predicated on the fact that metabolic rate and temperature are directly (though not linearly) related. Oxygen consumption (VO2 ) decreases approximately 5% to 7% for every 1°C decrease in temperature. Expressed differently, a 10°C decrement in temperature from 37°C (termed Q10 ) will cause a twofold to threefold decrease in oxygen consumption. At lower temperatures, Q10 may differ and probably exhibits organ specificity. For the brain, the Q10 for the temperature range 27°C to 17°C is 5 to 6. In other words, in the brain the decrement in VO2 is dramatically greater at lower temperatures.[229] Hypothermia with a lower VO2 allows lower flow rates on CPB and potentially less myocardial collateral flow and myocardial rewarming (systemic temperatures of 27°C to 32°C are greater than myocardial temperatures of 12°C to 15°C). Temperature and temperature gradients (inflow versus outflow, core representing high tissue perfusion versus peripheral representing low tissue perfusion) are monitored continuously during CPB. However, temperature monitoring represents but one component of monitoring, whose overall objective is to monitor the adequacy of tissue oxygenation. In addition to temperature monitoring, pump flow rates, in-line arterial and venous blood gas analysis, and oxygen saturation measurements should be continuously monitored during CPB to assess the efficacy of tissue oxygenation. Temperature gradients are additionally important for two reasons. First, hypothermia increases the solubility of gases, and higher temperatures increase the rate at which blood gases come out of solution. Thus, an excessive temperature gradient (e.g., between venous outflow blood and arterial inflow blood) will accelerate the rate at which dissolved blood gases come out of solution, with the potential for bubble formation and the thromboembolic consequences thereof. It is thus recommended that temperature gradients not exceed 10°C. Second, large temperature gradients at the end of CPB between areas of high and low perfusion are indicative of inadequate rewarming and portend hypothermia after CPB as re-equilibration occurs. The consequences of this could include coagulopathy.
Absolute temperature, as well as temperature gradients, should be monitored closely during the entire CPB procedure, but especially during rewarming. The beneficial influence of hypothermia on VO2 during the abnormal cardiovascular conditions inherent in CPB also, unfortunately, operates in the converse situation. During rewarming on CPB, abnormal pressure, flow, and flow patterns are still present, but VO2 increases with increases in temperature, especially in well-perfused organs such as the brain. The practice of warming inflow blood to higher than 37°C (even with acceptable gradients) with a parallel increase in brain temperature and the cerebral metabolic rate of oxygen (CMRO2 ) may have deleterious neurologic complications postoperatively. The cost of restraining
Most centers maintain mean arterial pressure (MAP) between 40 and 60 mm Hg during CPB by using nonpulsatile flow rates of 50 to 60 mL/kg. However, variations in each of these parameters have been the subject of extensive research, for example, outcomes in patients managed with high versus low MAP during CPB and outcomes in patients managed with pulsatile versus nonpulsatile flow. The inability to demonstrate benefits from approaches that seem more physiologic and do have a sound theoretical basis reflects our inability to simulate physiologic conditions on CPB and variability in the patient's underlying condition.
Surprisingly, the optimum MAP on CPB is not universally agreed on. Low MAP refers to levels between 40 and 60 mm Hg, whereas high MAP refers to levels greater than 80 mm Hg. This may reflect the primary importance of flow rates (identical to cardiac output) during CPB. At any particular temperature, MAP and flow rates influence organ perfusion, including myocardial and cerebral perfusion. Although the aorta is cross-clamped and there is no coronary blood flow, the myocardium may still be perfused by collateral channels during CPB and aortic cross-clamping. Indeed, the contribution of collateral flow is likely to be greater in the presence of severe obstructive coronary artery disease. Collateral flow in particular may be perfusion pressure-dependent. The higher the MAP, the greater the collateral flow and thus the propensity for myocardial rewarming and obscuring of the surgical field. Conversely, some investigators argue that increased collateral flow to areas of poorly protected myocardium distal to obstructive lesions may be beneficial. No study has thus far shown any benefit of high versus low MAP on myocardial protection.
Although PCO2 and CMRO2 are the main determinants of cerebral blood flow, MAP is clearly important, and its importance is probably amplified in the presence of cerebral vascular disease, which is not uncommon with coexisting coronary artery disease. Several studies have attempted to determine the influence of MAP during CPB on neurologic outcome. Some past studies demonstrated a beneficial effect of higher MAP on neurologic events,[232] whereas others did not.[233] However, more recent data[234] support the notion that higher MAP (80 to 100 mm Hg) significantly improves neurologic outcome, and most centers now incorporate higher MAP into their practice in patients with documented cerebral vascular disease.
In theory, pulsatile blood flow should improve capillary and thus tissue perfusion. However, the techniques currently available to simulate the physiologic condition fall far short of that objective. Moreover, the techniques are expensive and complex. Numerous studies have examined the influence of pulsatility on outcome and on renal and neurologic complications in particular. Renal blood flow and urinary output seem to be better preserved with pulsatile flow,[235] [236] [237] and one study suggested that pulsatile flow was preferable in patients with impaired renal function.[238] Animal model studies have demonstrated improved cerebral blood flow with pulsatile flow, but this finding has not been shown to translate into clinical benefit for patients.[239] [240] Overall, the outcome data have failed to demonstrate a clinical benefit of pulsatile flow, and nonpulsatile techniques represent standard practice.
The initiation of CPB causes an abrupt decrease in hematocrit and oncotic pressure when crystalloid priming solutions are used. The decrease in oncotic pressure alters microvascular Starling forces and causes efflux of fluid from the intravascular to the extravascular compartments. The end result is generalized tissue swelling. Despite a sound theoretical basis, the addition of albumin to priming solutions does not confer clinical benefit[241] and is not standard practice.
The abrupt decrease in hematocrit and thus viscosity at the onset
of CPB is probably an important contributor to the hypotension observed at this time.
However, with progressive hypothermia, viscosity increases and in parallel so does
MAP. The influence of hematocrit on blood viscosity was first quantified in the
form of the Rand equation[242]
[243]
:
μx
− μP (1 + 0.025 H + 0.000735
H2
)
Even more important than the influence of viscosity on shear rate and MAP ( Fig.
50-37
) is its effect on flow and
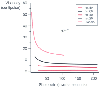 Figure 50-37
Blood rheology: relationship between viscosity and shear
rate in the blood of healthy volunteers reconstituted to hematocrit (hct) values
of 0%, 20%, 40%, 60%, and 80% at a temperature of 37°C. (Redrawn from
Rand PW, Lacomb E, Hunt HE, Austin WH: Viscosity of normal human blood under normothermic
and hypothermic conditions. J Appl Physiol 19:117, 1964.)
Figure 50-37
Blood rheology: relationship between viscosity and shear
rate in the blood of healthy volunteers reconstituted to hematocrit (hct) values
of 0%, 20%, 40%, 60%, and 80% at a temperature of 37°C. (Redrawn from
Rand PW, Lacomb E, Hunt HE, Austin WH: Viscosity of normal human blood under normothermic
and hypothermic conditions. J Appl Physiol 19:117, 1964.)
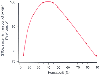 Figure 50-38
Relationship between systemic transport of oxygen (STO2
)
and hematocrit (%). Oxygen delivery is maximum in the hematocrit range of 35% to
45%. (Redrawn from Gordon RJ, Kavin MB, Dalcoff GR: Blood rheology. In
Thomas CC [ed]: Cardiovascular Physiology for Anesthesiologists. Springfield, IL,
Charles C Thomas, 1979, pp 27–71.)
Figure 50-38
Relationship between systemic transport of oxygen (STO2
)
and hematocrit (%). Oxygen delivery is maximum in the hematocrit range of 35% to
45%. (Redrawn from Gordon RJ, Kavin MB, Dalcoff GR: Blood rheology. In
Thomas CC [ed]: Cardiovascular Physiology for Anesthesiologists. Springfield, IL,
Charles C Thomas, 1979, pp 27–71.)
The biochemical basis of changes in the pH of neutrality of water, the pH of acids and their conjugate bases, and the pKa of buffer systems and the optimal acid-base management strategy during hypothermic CPB represent frequent board examination questions, are the basis of much debate, and have been the focus of several research studies. The biochemical basis of hypothermia-related acid-base changes is discussed in detail elsewhere,[244] [245]
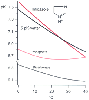 Figure 50-39
Changes in the dissociation constant (pK)
of CO2
-bicarbonate, phosphate, and imidazole with temperature. The 0.5
pK of water, or neutrality, is also shown. (Redrawn from Rahn H: Body temperature
and acid-base regulation. Pneumonologie 151:87, 1974.)
Figure 50-39
Changes in the dissociation constant (pK)
of CO2
-bicarbonate, phosphate, and imidazole with temperature. The 0.5
pK of water, or neutrality, is also shown. (Redrawn from Rahn H: Body temperature
and acid-base regulation. Pneumonologie 151:87, 1974.)
If the pH of the milieu containing this buffer system also increases with hypothermia, the propensity for imidazole to alter its acid-base ratios (or the ratio of either to the total concentration of imidazole) will remain unchanged, or static—hence the derivation of the term "alpha stat." Conversely, if the pH is kept at 7.4 irrespective of temperature (by adding carbon dioxide), hypothermia will cause acidosis. Note that based on the aforementioned assertions, a pH of 7.4 at any temperature less than 37°C represents acidosis. This will change the alpha number of imidazole and thus alter its buffering capacity and the function of proteins and enzymes of which it is an important and integral component. The issue of whether it is desirable or otherwise to promote enzyme and therefore cellular function under conditions of hypothermia relates to the unanswered question of whether homeotherms behave like hibernators or ectotherms under hypothermic conditions.[246]
The potential impact of hypothermia-induced acid-base changes is likely to be greater at the extremes of hypothermia, for example, in the myocardium during aortic cross-clamping or during deep hypothermic circulatory arrest (DHCA). However, during aortic cross-clamping
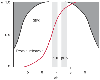 Figure 50-40
An enlarged view of the ionization window showing the
range of intracellular muscle pH values of ectotherms at 25°C, which is close
to the neutral pH of water at that temperature. The typical range for the blood
value is also shown, as well as a mean value of the dissociation curve for the protein
buffer imidazole in histidine. At intracellular pH, its dissociation has a value
of about 0.55. (Redrawn from Rahn H, Reeves RB, Howell BJ: Hydrogen ion
regulation, temperature, and evolution. Am Rev Respir Dis 112:165–172, 1975.)
Figure 50-40
An enlarged view of the ionization window showing the
range of intracellular muscle pH values of ectotherms at 25°C, which is close
to the neutral pH of water at that temperature. The typical range for the blood
value is also shown, as well as a mean value of the dissociation curve for the protein
buffer imidazole in histidine. At intracellular pH, its dissociation has a value
of about 0.55. (Redrawn from Rahn H, Reeves RB, Howell BJ: Hydrogen ion
regulation, temperature, and evolution. Am Rev Respir Dis 112:165–172, 1975.)
Experimental laboratory data and clinical data in nonsurgical patients[250] indicate that hyperglycemia exacerbates neurologic injury. Moreover, aggressive glucose homeostasis is associated with improved outcome in ICU patients.[251] Hence, it is reasonable and prudent to promote glucose control in a patient population that is at increased risk of neurologic injury and invariably becomes hyperglycemic as part of the stress response to surgery and CPB. This occurs even in nondiabetic patients.
|
|
|
|
|
|
|
|
|
|
|
|
|