Myocardial Protection
In its broadest, truest sense, myocardial protection refers to
all interventions undertaken in the preoperative, intraoperative, and postoperative
periods that optimize myocardial oxygen supply and demand. As such, this definition
incorporates all medical and interventional cardiology strategies and all manipulations
of the determinants of oxygen supply and demand undertaken by the cardiac anesthesiologist.
However, the more common and yet much narrower use of the term "myocardial protection"
in cardiac surgery refers only to interventions undertaken during CPB and specifically
during the period of aortic cross-clamping. Although cardiac procedures are now
commonly performed without CPB and aortic cross-clamping, most procedures still use
these latter techniques. Moreover, the broader concept of myocardial protection
is still applicable to off-pump procedures. For example, the value of β-blockers
in these circumstances may have more to do with myocardial oxygen supply/demand ratios
than to optimizing the surgical field for the surgeon.
The earliest cardiac surgical procedures used venous inflow occlusion
techniques. However, time limitations precluded performing anything but the simplest
procedures. With the development of CPB, hypothermia became the primary technique
of tissue protection in general and myocardial protection in particular. However,
the limitations of this approach became evident quickly, and further advances in
myocardial protection techniques (and as a corollary, the complexity of the cardiac
surgical procedure undertaken) awaited the introduction of cardioplegia by the St.
Thomas group in 1975.[215]
[216]
Ironically, the use of high potassium solutions to induce diastolic arrest was introduced
2 decades earlier (about the time that CPB was first described),[217]
but it was not pursued clinically because of the finding of focal myocardial necrosis
probably resulting from very high potassium concentrations. Blood cardioplegia was
first described only as recently as 1978.[218]
The overall objective of myocardial protection techniques is to
minimize oxygen consumption and the consequences of oxygen deprivation during aortic
cross-clamping. Systemic hypothermia (with its influence on collateral flow to the
heart), topical hypothermia, and above all, some combination of the available cardioplegic
techniques are used to that end. Importantly, however, it is now also well recognized
that the consequences of aortic cross-clamping result not only from ischemia during
this period but also from reperfusion injury at the end of aortic cross-clamping.
A detailed description of the metabolic, signaling, and cellular consequences of
ischemia-reperfusion injury is beyond the scope of this chapter. However, it is
important to remember that ischemia causes rapid depletion of ATP, increased anaerobic
glycolysis, accumulation of lactic acid, eventual inability to maintain cellular
ionic gradients, intracellular accumulation of osmotically active ions (including
calcium), cell swelling, and, in the continued absence of oxygen, eventual cell death.
Reperfusion of ischemic myocytes (if they are still viable) causes additional cellular
injury. Production of ROS in particular, leukocyte activation, perturbations in
eicosanoid metabolism, and further accumulations in intracellular calcium concentrations
are now recognized to be mechanistically important contributors to this component
of cellular injury.
Clinically, reperfusion injury can be manifested as dysrhythmia
or a stunned myocardium. Mechanistically, stunning is thought to be caused by oxyradical-induced
myofibrillar damage (with resultant impaired responses to calcium), as well as intracellular
calcium overload.[219]
Ongoing attempts to improve
the efficacy of cardioplegia are motivated by our understanding of the mechanisms
underlying ischemia-reperfusion injury and the desire to successfully interrogate
these mechanisms. For example, manipulating tonicity to attenuate cellular swelling,
altering buffering capacity to decrease acidosis, optimizing calcium levels to limit
intracellular calcium overload yet maintain contractile function, and adding free
radical scavengers to decrease oxyradical injury represent efforts to attenuate ischemia-reperfusion
injury. Although these
TABLE 50-14 -- Myocardial oxygen consumption (at 37°C) for different work and electrical
conditions
|
Myocardial Oxygen Consumption
(mL/100 g/min) |
|
Beating (full, perfused) |
10.0 |
|
Beating (empty, perfused) |
5.5 |
|
Fibrillating (empty, perfused) |
6.5 |
|
K+
cardioplegia (empty, cross-clamp) |
1.0 |
|
Data from Sarnoff SJ, Gilmore JP, McDonald RH Jr,
et al: Relationship between myocardial K+
balance, O2
consumption,
and contractility. Am J Physiol 211:361–375, 1966; and Sarnoff SJ, Gilmore
JP, Daggett WM, et al: Myocardial dynamics, contractility, O2
consumption,
and K+
balance during paired stimulation. Am J Physiol 211:376–386,
1966. |
are important and ongoing areas of study, the original rationale for using cardioplegic
solutions was based on the profound influence of (1) diastolic arrest (versus a beating
heart), (2) temperature, and (3) a full versus empty heart on myocardial oxygen requirements
( Table 50-14
and Table
50-15
, Fig. 50-36
).
[220]
[221]
The literature on cardioplegic solutions can be confusing. Consider
that in addition to blood cardioplegia, there are several variations in crystalloid
cardioplegia. Moreover, cardioplegia can be delivered antegradely or retrogradely,
or in combination, and through saphenous vein grafts after the completion of distal
anastomoses. Retrograde cardioplegia is technically more difficult, should be delivered
with pressures less than 40 mm Hg, does not necessarily deliver cardioplegia to the
same capillary bed as antegrade cardioplegia does, and may be the victim of variable
venous anatomy, for example, the left superior vena cava draining into the coronary
sinus or the tip of the cardioplegia cannulas proximal to the great cardiac vein.
Nevertheless, it may be the preferred technique in the presence of aortic incompetence
or severe coronary obstruction. The duration, quantity, and frequency of cardioplegia
administration vary from surgeon to surgeon and study
TABLE 50-15 -- Influence of temperature on myocardial oxygen consumption for different work
and electrical conditions
|
Myocardial Oxygen Consumption
(mL/100 g/min) |
|
37°C |
32°C |
28°C |
22°C |
|
Beating (empty) |
5.5 |
5.0 |
4.0 |
2.9 |
|
Fibrillating (empty) |
6.5 |
3.8 |
3.0 |
2.0 |
|
K+
cardioplegia |
1.0 |
0.8 |
0.6 |
0.3 |
|
Data from Sarnoff SJ, Gilmore JP, McDonald RH Jr,
et al: Relationship between myocardial K+
balance, O2
consumption,
and contractility. Am J Physiol 211:361–375, 1966; and Sarnoff SJ, Gilmore
JP, Daggett WM, et al: Myocardial dynamics, contractility, O2
consumption,
and K+
balance during paired stimulation. Am J Physiol 211:376–386,
1966. |
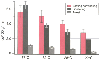 Figure 50-36
Myocardial oxygen uptake in different states of temperature
and workload. (Redrawn from Buckberg GD, Brazier JR, Nelson RL, et al:
Studies of the effects of hypothermia on regional myocardial blood flow and metabolism
during cardiopulmonary bypass. I. The adequately perfuse beating, fibrillating,
and arrested heart. J Thorac Cardiovasc Surg 73:87–94, 1977.)
Figure 50-36
Myocardial oxygen uptake in different states of temperature
and workload. (Redrawn from Buckberg GD, Brazier JR, Nelson RL, et al:
Studies of the effects of hypothermia on regional myocardial blood flow and metabolism
during cardiopulmonary bypass. I. The adequately perfuse beating, fibrillating,
and arrested heart. J Thorac Cardiovasc Surg 73:87–94, 1977.)
to study. Cardioplegia techniques may or may not be combined with topical hypothermia
(with the attendant risk of phrenic nerve injury), and the level of systemic hypothermia
used varies. More recently, warm blood induction and reperfusion cardioplegia represent
additional variables.
Finally, the patient's underlying disease may modulate delivery
of cardioplegia (e.g., stenosed coronary vessels) or its efficacy (hypertrophied
myocardium). However, overall, the most recent data indicate that blood cardioplegia
is used in 80% to 85% of cardiac surgery procedures in the United States and that
the retrograde approach is used either alone or in combination in 45% of cases.[222]
[223]
Although cardioplegic solutions are administered
with the objective of producing uniform myocardial temperatures of 12°C to 15°C,
some evidence suggests that warm induction and reperfusion improves clinically relevant
outcome parameters.[224]
[225]
Many studies have compared blood and crystalloid cardioplegia
and have been reviewed.[226]
Several recent studies
have demonstrated either the superiority of blood cardioplegia over crystalloid cardioplegia
or have failed to show a difference between the two. Intuitively, we would predict
that in patients with well-preserved myocardial function who are subjected to less
protracted and complex procedures, the type and route of cardioplegia delivery probably
make little difference. The converse may also be true. Indeed, two relatively recent
studies illustrate just that. In patients with well-preserved myocardial function,
the type of cardioplegia made no difference as assessed by the magnitude of enzyme
leak.[227]
In contrast, in patients with ejection
fractions less than 40%, blood cardioplegia was superior to crystalloid cardioplegia
as determined by enzyme leak, function parameters, and rhythm and conduction abnormalities.
[228]
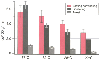 Figure 50-36
Myocardial oxygen uptake in different states of temperature
and workload. (Redrawn from Buckberg GD, Brazier JR, Nelson RL, et al:
Studies of the effects of hypothermia on regional myocardial blood flow and metabolism
during cardiopulmonary bypass. I. The adequately perfuse beating, fibrillating,
and arrested heart. J Thorac Cardiovasc Surg 73:87–94, 1977.)
Figure 50-36
Myocardial oxygen uptake in different states of temperature
and workload. (Redrawn from Buckberg GD, Brazier JR, Nelson RL, et al:
Studies of the effects of hypothermia on regional myocardial blood flow and metabolism
during cardiopulmonary bypass. I. The adequately perfuse beating, fibrillating,
and arrested heart. J Thorac Cardiovasc Surg 73:87–94, 1977.)