|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Indications for heart transplantation are outlined in Table 50-8 . Although the causes of end-stage heart failure are protean (ischemia, end-stage valvular disease, viral, post-irradiation, post-chemotherapy, idiopathic), all patients will be receiving maximal medical therapy and various combinations of converting-enzyme inhibitors, anticoagulants, antiplatelet agents, β-blockers, calcium antagonists, diuretics, digoxin, amiodarone, and other drugs.
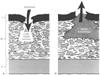 Figure 50-26
Proposed mechanisms of initiation of aortic dissection.
In both cases, cystic medial necrosis is present. In A,
an intimal tear is the initial event and is allowing aortic blood to enter the media.
In B, the primary event is hemorrhage into the media,
with secondary rupture of the overlying intima. A, adventitia; I, intima; M, media.
Figure 50-26
Proposed mechanisms of initiation of aortic dissection.
In both cases, cystic medial necrosis is present. In A,
an intimal tear is the initial event and is allowing aortic blood to enter the media.
In B, the primary event is hemorrhage into the media,
with secondary rupture of the overlying intima. A, adventitia; I, intima; M, media.
Pulmonary vascular congestion, pulmonary hypertension, and depending on the duration and severity, elevated pulmonary vascular resistance are inevitable and intrinsic features of end-stage heart failure. Severe pulmonary hypertension with elevated pulmonary vascular resistance has, depending on its magnitude, been a contraindication to heart transplantation. The upper limits of acceptability vary among centers. An upper limit of pulmonary vascular resistance of 6 Wood units is used at most centers.[160] (Wood resistance units are calculated as the quotient of the pulmonary vascular pressure gradient, measured in mm Hg, divided by flow, measured in L/m.) The degree of reversibility of elevated pulmonary vascular resistance is
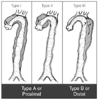 Figure 50-27
Commonly used classification systems for aortic dissection.
(From Isselbacher EM, Eagle KA, Desanctis RW: Diseases of the aorta. In
Braunwald E [ed]: Heart Disease. A Textbook of Cardiovascular Medicine, 5th ed.
Philadelphia, WB Saunders, 1980, pp 1546–1581.)
Figure 50-27
Commonly used classification systems for aortic dissection.
(From Isselbacher EM, Eagle KA, Desanctis RW: Diseases of the aorta. In
Braunwald E [ed]: Heart Disease. A Textbook of Cardiovascular Medicine, 5th ed.
Philadelphia, WB Saunders, 1980, pp 1546–1581.)
|
|
Site of Origin and Extent of Aortic Involvement |
|---|---|
| DeBakey | |
| Type I | Originates in the ascending aorta and propagates at least to the aortic arch and often beyond it distally |
| Type II | Originates in and is confined to the ascending aorta |
| Type III | Originates in the descending aorta and extends distally down the aorta or, rarely, retrogradely into the aortic arch and ascending aorta |
| Stanford | |
| Type A | All dissections involving the ascending aorta, regardless of the site of origin |
| Type B | All dissections not involving the ascending aorta |
| Descriptive | |
| Proximal | Includes DeBakey types I and II or Stanford type A |
| Distal | Includes DeBakey type III or Stanford type B |
| From Isselbacher EM, Eagle KA, Desanctis RW: Diseases of the aorta. In Braunwald E (ed): Heart Disease. A Textbook of Cardiovascular Medicine, 5th ed. Philadelphia, WB Saunders, 1980, pp 1546–1581. | |
| End-state heart disease not amenable to other medical or surgical therapy |
| Class III–IV symptoms with optimal medical therapy and prognosis for 1-year survival less than 50% |
| Age ≤ 60–65 |
| Healthy apart from heart disease |
| Emotionally stable, well motivated to resume active lifestyle |
| Compliant with medical advice |
| Supportive family/companions willing and able to make similar long-term commitments |
| From Kasper EK, Achuff SC: Clinical evaluation of potential heart transplant recipients. In Baumgartner W, Reitz B, Kasper E, Theodore J (eds): Heart and Lung Transplantation, 2nd ed. Philadelphia, WB Saunders, 2002, pp 57–63. |
Increasingly, patients are placed on mechanical circulatory support systems as a bridge to transplantation, which has obvious implications for the timing and difficulty of surgery. These patients pose a management dilemma. The nature of the surgery makes it desirable to use aggressive antifibrinolytic therapy such as aprotinin. Although re-exposure to aprotinin places patients at increased risk for antibody/immune-mediated reactions, clinical experience and antibody data indicate that such reactions are less frequent than would be predicted, especially as the interval between exposures increases.
Common causes of cardiac tamponade are listed in Table
50-9
. Though often and understandingly viewed by the anesthesiologist
as an acute life-threatening event requiring urgent surgical intervention, cardiac
tamponade can have a wide spectrum of clinical manifestations ranging from mild to
severe. The rapidity of fluid accumulation in the pericardium and the related variable
of pericardial sac compliance are the primary determinants of the physiologic impairments
that occur with tamponade ( Fig. 50-28
).
Acute accumulation of a relatively small volume of fluid in a noncompliant "compartment"
can lead to rapid cardiovascular collapse. In contrast, larger
| Hemorrhagic pericarditis caused by |
| Aortic dissection |
| Ventricular free wall rupture after myocardial infarction |
| Anticoagulant-induced hemopericardium |
| Trauma (stab wounds, central venous catheters) |
| Cardiac surgery |
| Uremic pericarditis |
| Neoplastic pericarditis (especially mesothelioma or lymphoma) |
| Serous pericarditis (rheumatoid disorders, irradiation, viral infection) |
| From MacVeigh I: Anesthesia for the surgical management of pericardial disease. In Thys DM (ed): Textbook of Cardiothoracic Anesthesiology. New York, McGraw-Hill, 2001, pp 630–643. Copyright © by McGraw-Hill, Inc. Used by permission of McGraw-Hill Book Company. |
 Figure 50-28
Pressure-volume relationship of the pericardium in the
presence of pericardial effusion. A, hyperacute cardiac tamponade (gunshot or stab
wounds to the heart); B, subacute cardiac tamponade—the effusion developed
over a period of a few days; C, effusion that has developed over a period of several
weeks to months; D, chronic effusive pericarditis—pericardial pressure is slightly
elevated but does not cause major hemodynamic impairment. (From Smith T:
Cardiovascular Therapeutics: A Companion to Braunwald's Heart Disease. Philadelphia,
WB Saunders, 1996, p 774.)
Figure 50-28
Pressure-volume relationship of the pericardium in the
presence of pericardial effusion. A, hyperacute cardiac tamponade (gunshot or stab
wounds to the heart); B, subacute cardiac tamponade—the effusion developed
over a period of a few days; C, effusion that has developed over a period of several
weeks to months; D, chronic effusive pericarditis—pericardial pressure is slightly
elevated but does not cause major hemodynamic impairment. (From Smith T:
Cardiovascular Therapeutics: A Companion to Braunwald's Heart Disease. Philadelphia,
WB Saunders, 1996, p 774.)
Under normal conditions, venous return to the heart is facilitated by the decrease in intracavity pressure that occurs post-systole. Fluid accumulation around the heart decreases and eliminates the transmural distending pressure that promotes cardiac filling. If severe, diastolic pressures increase. Moreover, diastolic pressures in the atria and ventricles tend to equalize. Impaired cardiac filling results in impaired cardiac output and activation of reflex neurohumoral mechanisms (autonomic nervous system, catecholamine release, vasopressin release, activation of the renin-angiotensin system) in an attempt to maintain adequate organ perfusion. Predictably, hypovolemia exacerbates the hemodynamic effects of tamponade. As a corollary, optimizing volume status, as well as having access to allow one to accomplish this, is a key feature of the perioperative management of these patients. Moreover, anesthetic regimens should be designed to use pharmacological tools that minimally attenuate (as much as possible) the sympathetic response that is the essential mechanism by which cardiac output and blood pressure are maintained (albeit inadequately) in these patients.
Constrictive pericarditis is most frequently idiopathic or viral. Other causes include radiation therapy, post-cardiac surgery, post-myocardial infarction (Dressler's syndrome), connective tissue disorders, and renal failure. As with tamponade, diastolic filling of the heart is restricted in constrictive pericarditis. Depending on the cause, level of progression, and severity of the process, varying degrees of pericardial calcification, pericardial effusion, and myocardial and/or coronary involvement may be present. In severe cases, the latter may be manifested as myocardial atrophy (and thus systolic dysfunction), whereas coronary involvement is probably mediated by scar-induced compression of the coronary arteries. The symptoms and signs of constrictive pericarditis are a function of impaired cardiac output (fatigue, malaise) and limitations in venous return to the left (dyspnea, cough) and/or the right (enlarged liver, ascites) sides of the heart. As in tamponade, intracavitary pressure measurements reveal equalization of diastolic pressures, but a more pronounced decrease in pressure in early diastole (corresponding to a pronounced y descent on CVP), thereby giving rise to the so-called square root sign ( Fig. 50-29 ).
Primary cardiac tumors are rare.[161] [162] Among primary cardiac tumors, benign tumors are much more common, and of benign tumors, myxomas are by far the most common. Approximately 85% of these usually solitary tumors occur in the left atrium.
Cardiac tumors may be manifested clinically in one or more of three ways: (1) nonspecific systemic features, (2) thromboembolic phenomena, and (3) local cardiac effects. Nonspecific systemic manifestations include fever, malaise, cachexia, arthralgia, rash, and behavioral changes. Mechanistically, there is good evidence to suggest that interleukin-6 synthesis by tumor tissue mediates
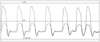 Figure 50-29
Simultaneous recordings of right and left ventricular
pressure showing "dip and plateau" waveforms and equalization of right and left ventricular
diastolic pressure. (From Baim DS, Grossman W: Cardiac Catheterization,
Angiography, and Intervention, 5th ed. New York, Lippincott Williams & Wilkins,
1995 p 419.)
Figure 50-29
Simultaneous recordings of right and left ventricular
pressure showing "dip and plateau" waveforms and equalization of right and left ventricular
diastolic pressure. (From Baim DS, Grossman W: Cardiac Catheterization,
Angiography, and Intervention, 5th ed. New York, Lippincott Williams & Wilkins,
1995 p 419.)
|
|
|
|
|
|
|
|
|
|
|
|
|