Leukoreduction of Red Blood Cell Transfusions
One of the most dramatic changes in transfusion medicine in the
past 5 years has been the universal use of leukoreduced PRBCs internationally, which
includes Western Europe, the United Kingdom, and Canada. In the United States as
of 2004, more than 50% of the PRBCs given are leukoreduced. What is the logic on
which the increased use of leukoreduced blood is based?
There are some clear indications for leukoreduced blood. The
chances of a febrile reaction can be reduced, especially in patients who are already
alloimmunized from pregnancy. The risk of HLA alloimmunization from blood transfusions
can be reduced, which would be especially helpful in minimizing refractoriness to
platelet transfusions, and the risk of CMV can be reduced by using leukoreduced blood.
Universal leukoreduction has been seriously considered or implemented
because of some anticipated benefits, including decreased transmission of vCJD, leukocyte-induced
immunomodulation, and even decreased postoperative mortality. In 2001, the case
for and against universal leukoreduction was debated.[131]
As of 2003, these anticipated benefits were not confirmed, despite numerous studies
attempting to do so.[132]
As nicely summarized
by Corwin and AuBuchon,[133]
a "may help, won't
hurt" approach has been used to justify universal leukoreduction. However,
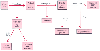 Figure 47-10
Scheme for separation of whole blood for component therapy.
Figure 47-10
Scheme for separation of whole blood for component therapy.
this approach can be expensive (about $500 million in the United States). Bacterial
contamination of platelets, TRALI, and acute hemolytic reactions cause more morbidity
and mortality that would not be significantly helped by leukoreduction (see Table
47-7
). Nevertheless, universal leukoreduction is the direction in which
transfusion medicine is going.
 Figure 47-10
Scheme for separation of whole blood for component therapy.
Figure 47-10
Scheme for separation of whole blood for component therapy.