|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Much of the previous discussion assumed the use of a non-rebreathing system and a fixed inspired concentration of anesthetic. Although this approach does not invalidate the principles described earlier, it also does not reflect the variety of approaches applied in practice. Practice often deviates in two ways. Most anesthetists use lower inflow (fresh gas flow) rates to provide a more economical delivery of anesthesia, and most anesthetists apply a constant alveolar, rather than a constant inspired, concentration because a constant alveolar concentration reflects a constant level of anesthesia.
A low inflow rate results in several advantages and a few disadvantages, with the latter particularly applying to kinetics. The advantages of low inflow administration (i.e., fresh gas flows of less than one half of the minute volume, usually less than 2 L/min) or closed-circuit anesthesia (i.e., delivery of gases in amounts just sufficient to replace the gases—oxygen and anesthetic—removed by the patient) include lower cost, increased humidification, reduced heat loss, decreased release of anesthetic to the environment, and better capacity to assess physiologic variables, such as ventilation. On the debit side, there is more concern about oxygen levels (especially if nitrous oxide is used, but the patient also contributes nitrogen from stores in the body, and such nitrogen can slightly decrease the inspired concentration of oxygen), about increasing concentrations of carbon monoxide, and about rebreathing of toxic products from the breakdown of volatile anesthetics. Regarding this last point, carbon dioxide absorbents can degrade halothane and sevoflurane to toxic unsaturated compounds.[75] [76] However, the debit of most immediate concern is the lack of control that low flows and, especially, closed circuits offer.
The use of a closed circuit represents an extreme of anesthetic administration, one infrequently employed because few systems completely eliminate leakage of gas from the circuit. Anesthetists often apply a deliberate leak of approximately 200 mL/min by sampling gases for oxygen, carbon dioxide, and anesthetic analyses.
Usually, closed-circuit anesthesia requires replacement of three gases: oxygen, nitrous oxide, and a potent
Uptake of potent anesthetics may be estimated from the values (constants) obtained by Yasuda and colleagues[11] [12] in humans. These values may be applied to obtain uptake at a constant alveolar concentration, such as at an alveolar concentration equal to the MAC. Figure 5-16 reveals parallel shapes for each anesthetic, shapes dictated by the perfusion and solvent characteristics of the three major tissue compartments plus intertissue diffusion. A large initial uptake rapidly decreases to a much lower level in 5 to 10 minutes, reflecting the high initial uptake by the VRG (because of its large perfusion) and the rapid decrease in uptake by the VRG imposed by a low capacity for anesthetics relative to perfusion. The subsequent slower decrease primarily results from the longer time to
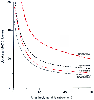 Figure 5-16
The uptake (in milliliters per minute) resulting from
the application of the constants calculated by Yasuda and colleagues[11]
[12]
from their measurements in human volunteers.
The application assumes that the alveolar concentration equals the minimum alveolar
concentration (MAC). Uptake is a function of MAC and solubility of the anesthetic
in blood and tissues. The fivefold higher MAC for desflurane compared with isoflurane
is offset by a threefold lower solubility, producing less than a twofold difference
in uptake at any time. Uptake for all anesthetics initially declines rapidly as
a function of the rate at which the vessel-rich tissue group equilibrates. Further
decline after 5 to 10 minutes is a function of the approach to equilibration of the
muscle group.
Figure 5-16
The uptake (in milliliters per minute) resulting from
the application of the constants calculated by Yasuda and colleagues[11]
[12]
from their measurements in human volunteers.
The application assumes that the alveolar concentration equals the minimum alveolar
concentration (MAC). Uptake is a function of MAC and solubility of the anesthetic
in blood and tissues. The fivefold higher MAC for desflurane compared with isoflurane
is offset by a threefold lower solubility, producing less than a twofold difference
in uptake at any time. Uptake for all anesthetics initially declines rapidly as
a function of the rate at which the vessel-rich tissue group equilibrates. Further
decline after 5 to 10 minutes is a function of the approach to equilibration of the
muscle group.
Although the curves for each anesthetic do not differ in shape, they have different positions. The height of each curve (i.e., uptake) is directly proportional to two factors: solubility and MAC. Solubility and MAC tend to move inversely, and this minimizes differences in uptake among anesthetics. For example, although the MAC for desflurane is five times that for isoflurane, desflurane's uptake is less than twice that of isoflurane because of desflurane's lower solubility in both blood and tissues.
Uptake may be estimated from the square-root-of-time rule, first proposed by Severinghaus[77] and expanded greatly by Lowe and associates[78] [79] in their classic descriptions of closed-circuit anesthesia. This rule states that uptake at any point in time may be estimated as uptake during the first minute of anesthesia divided by the square root of time in minutes. Making certain assumptions permits an estimate of uptake during the first minute. In general, uptake equals the product of blood solubility, cardiac output, and alveolar-to-venous anesthetic partial pressure difference. Several sources supply standard values for solubility and cardiac output, and the alveolar-to-venous anesthetic partial pressure difference may be estimated if we determine what alveolar partial pressure we wish and assume that venous anesthetic partial pressure is inconsequential. An inconsequential venous partial pressure is reasonable because no anesthetic can appear in venous blood before recirculation (about 0.5 minute), and even the anesthetic that appears is small because tissue uptake is maximal during the first minute. Isoflurane uptake in a normal adult can be estimated as 1.4 × 5400 × 0.0115, or 87 mL; 1.4 is the blood-gas partition coefficient, 5400 is a reasonable cardiac output, and 0.0115 is the MAC as a fraction of one atmosphere (1 atm). By 4 minutes, uptake would equal 87 mL/2; by 9 minutes, 87 mL/3; and by 64 minutes, 87 mL/8.
Hendrickx and coworkers[80] questioned the accuracy of the square-root-of-time rule, suggesting that the rule overestimates the decrease in uptake with the passage of time. This matter continues to be debated, [81] but Hendrickx's argument probably is correct: the square-root-of-time rule (an empirical observation) probably provides an imperfect description of uptake.
Replacement of anesthetic taken up may be accomplished by infusion of liquid anesthetic directly into the anesthetic circuit, either continuously or as boluses. A continuous infusion requires a pump of some sort, and an elegant solution uses a computer to direct a progressive decrease in infusion rate as a function of time. Bolus injection from a syringe has a neat simplicity but has two disadvantages. The circuit concentration modestly oscillates, and the anesthetist is required to remember when and how much to inject. A further disadvantage accrues to the injection of desflurane by pump or syringe. The high vapor pressure of desflurane (about 1 atm at room temperature) results in the unpredictable formation of bubbles of desflurane gas and a potential for a marked variability in the rate of infusion or injection, especially when smaller volumes of liquid must be injected.
An alternative solution to injection of liquid applies a conventional vaporizer, one capable of accurate delivery
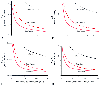 Figure 5-17
The ratio of delivered to alveolar concentrations (FD/FA)
required to sustain the alveolar concentration constant at, for example, the MAC,
is a function of several factors. First, it is determined by uptake (see Fig.
5-16
). Second, it is determined by rebreathing. The ratio therefore decreases
with increasing inflow rates (A to D).
Although these graphs have the same shape, notice the progressive decrease in the
scale on the ordinate as inflow rate increases. The lowest values for any anesthetic
result from a nonrebreathing system (i.e., when the inflow rate equals minute ventilation).
Figure 5-17
The ratio of delivered to alveolar concentrations (FD/FA)
required to sustain the alveolar concentration constant at, for example, the MAC,
is a function of several factors. First, it is determined by uptake (see Fig.
5-16
). Second, it is determined by rebreathing. The ratio therefore decreases
with increasing inflow rates (A to D).
Although these graphs have the same shape, notice the progressive decrease in the
scale on the ordinate as inflow rate increases. The lowest values for any anesthetic
result from a nonrebreathing system (i.e., when the inflow rate equals minute ventilation).
Figure 5-17A suggests one of the major difficulties associated with the closed-circuit approach: control. There is an enormous difference between delivered (i.e., vaporizer dial) and alveolar concentrations. The difference decreases with decreasing anesthetic solubility, but even with an anesthetic such as desflurane and even after the initial high-uptake period is passed, the dialed concentration markedly exceeds the alveolar concentration that it sustains. This means that changes in uptake (e.g., from the increase in cardiac output that may result from surgical stimulation) can cause considerable alterations in alveolar concentration unless the delivered concentration is altered. The alveolar concentration changes for two reasons. First, assuming a constant ventilation, the difference between the alveolar and inspired concentrations varies directly with uptake (e.g., a greater uptake
|
|
|
|
|
|
|
|
|
|
|
|
|