PHYSIOLOGIC EFFECTS
The physiologic effects of neuraxial blocks are often misinterpreted
as complications, which are highlighted by observers listing hypotension under complications
of the techniques. A clear distinction should be made between physiologic effects
of an anesthetic technique and complications that imply some harm to the patient.
[36]
This distinction is important to determine
the risk-benefit equation of the technique in question.
Cardiovascular Effects
The cardiovascular effects of neuraxial blocks are similar in
some ways to the combined use of intravenous α1
- and β-adrenergic
blockers: decreased heart rate and arterial blood pressure (see Chapter
16
). The sympathectomy that accompanies the techniques depends on the
height of the block, with the sympathectomy typically described as extending for
two to six dermatomes above the sensory level with spinal anesthesia and at the same
level with epidural anesthesia.[37]
This result
causes venous and arterial vasodilation, but because of the large amount of blood
in the venous system (approximately 75% of total blood volume), the venodilation
effect predominates because of the limited amount of smooth muscle in venules, whereas
the vascular smooth muscle on the arterial side of the circulation retains a considerable
degree of autonomous tone. After neuraxial block-induced sympathectomy, if normal
cardiac output is maintained, total peripheral resistance should decrease only 15%
to 18% in normovolemic healthy patients, even with near total sympathectomy. In
elderly patients with cardiac disease, systemic vascular resistance may decrease
almost 25% after spinal anesthesia, whereas cardiac output decreases only 10%.[38]
Heart rate during high neuraxial block typically decreases as a result of blockade
of the cardioaccelerator fibers arising from T1 to T4. The heart rate may decrease
as a result of a fall in right atrial filling, which decreases outflow from intrinsic
chronotropic stretch receptors located in the right atrium and great veins.[37]
The clinical question of what level of arterial blood pressure
decrease after neuraxial block is acceptable remains to be answered. It probably
will remain speculative because conducting ethical human investigations designed
to define a dose-response curve of decreased arterial blood pressure accompanying
neuraxial block will be difficult. That dilemma notwithstanding, it appears that
total body oxygen consumption in patients undergoing spinal anesthesia correlates
with extent of spinal anesthesia, providing a margin of safety for organ perfusion
unavailable with non-neuraxial techniques.[39]
Some data are available to help determine the extent to which
arterial blood pressure should be allowed to decrease. Although there are methodologic
problems with the study,
*
Kety and colleagues[40]
demonstrated that producing spinal anesthesia to midthoracic levels with procaine,
even in patients with essential hypertension, resulted in a decrease in mean arterial
pressure of 26% (155 to 115 mm Hg) accompanied by only a 12% (52 to 46 mL/100 g/min)
decrease in cerebral blood flow. When the level of spinal anesthesia was purposely
increased to produce higher levels of block (T4) in normotensive and hypertensive
patients, the mean arterial pressure decreased by 32% (93 to 63 mm Hg) and 50% (158
to 79 mm Hg), respectively. Although cerebral blood flow was unchanged in the normotensive
group (45 to 46 mL/100 g/min), a 19% decrease occurred in the apparently untreated
hypertensive patients (46.5 to 37.5 mL/100 g/min).[41]
When coronary artery blood flow and myocardial metabolism were determined in humans
during spinal anesthesia to
*Cerebral blood
flow (CBF) was studied with nitrous oxide. Only global CBF was measured, and the
patients studied were severely hypertensive (i.e., average mean arterial blood pressure
was 155 mm Hg).
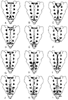 Figure 43-6
Anatomic variants of sacrum and sacral hiatus. Sacral
hiatus variants. A, Normal. B,
Longitudinal slitlike hiatus. C, Second midline hiatus.
D, Transverse hiatus. E,
Large hiatus with absent cornua. F, Transverse hiatus
with absent coccyx and two prominent cornua and two proximal "decoy hiatuses lateral
to the cornua." G–I, Large midline defects
contiguous with sacral hiatus. J–L, Enlarged
longitudinal hiatuses, each with an overlying decoy hiatus. (From Willis
RJ: Caudal epidural block. In Cousins MN, Bridenbaugh
PO [eds]: Neural Blockade in Clinical Anesthesia and Management of Pain, 2nd ed.
Philadelphia, JB Lippincott, 1988, p 365.)
Figure 43-6
Anatomic variants of sacrum and sacral hiatus. Sacral
hiatus variants. A, Normal. B,
Longitudinal slitlike hiatus. C, Second midline hiatus.
D, Transverse hiatus. E,
Large hiatus with absent cornua. F, Transverse hiatus
with absent coccyx and two prominent cornua and two proximal "decoy hiatuses lateral
to the cornua." G–I, Large midline defects
contiguous with sacral hiatus. J–L, Enlarged
longitudinal hiatuses, each with an overlying decoy hiatus. (From Willis
RJ: Caudal epidural block. In Cousins MN, Bridenbaugh
PO [eds]: Neural Blockade in Clinical Anesthesia and Management of Pain, 2nd ed.
Philadelphia, JB Lippincott, 1988, p 365.)
T4 in hypertensive and normotensive patients, decreases in coronary blood flow (153
to 74 mL/100 g/min) paralleled the decrease in mean arterial blood pressure (119
to 62 mm Hg), and the percentage extraction of myocardial oxygen was unchanged (75%
to 72%). The extraction of oxygen was unchanged because myocardial work, as expressed
by myocardial use of oxygen, paralleled the decrease in mean arterial pressure and
coronary blood flow (16 to 7.5 mL/100 g/min).[42]
These data support the observations by Stanley and coworkers[39]
but still do not provide a patient-by-patient indication of the organ most at risk
of flow-related ischemia, suggesting further research in this area is required.
Human investigations are limited by cerebral and myocardial blood
flow methodologies that were not limiting factors when Sivarajan and associates[43]
investigated organ blood flow by means of microspheres in rhesus monkeys during spinal
anesthesia at the T10 and T1 levels. During T10 block, there was no significant
change in organ blood flow; during the T1 block, with a 22% decrease in mean arterial
pressure, cerebral and myocardial blood flows were insignificantly altered. Prevention
of decreases in mean arterial pressure of more than 30% has some basis, but it is
important to remember these data were collected in severely hypertensive, presumably
untreated patients. For normotensive and treated hypertensive patients, a wider
undocumented margin of safety probably exists.
After arterial blood pressure decreases to a level for which treatment
is believed necessary, ephedrine, a mixed adrenergic agonist, provides more appropriate
therapy for the noncardiac circulatory sequelae of neuraxial block than a pure α-adrenergic
agonist (see Chapter 18
)
unless the patient has a specific and defined blood pressure requirement.[44]
It has long been taught that the decrease in blood pressure after neuraxial block
can be minimized by administration of crystalloids intravenously before the block;
however this logic needs rethinking. When all the
data are considered, it appears that 250- to 2000-mL preblock hydration regimens
appear to temporarily increase preload and cardiac output without consistently increasing
arterial pressure or preventing hypotension.[16]
Is there any indication that epidural and spinal anesthesia differ
in the effect on arterial blood pressure? A common concept is that the decrease
in arterial blood pressure is more gradual and of less magnitude with epidural than
spinal anesthesia of comparable levels. Despite this belief, there is evidence that
when tetracaine (10 mg) spinal anesthesia was compared with lidocaine (20 to 25 mL
of a 1.5% solution) epidural anesthesia, there was a larger decrease in arterial
blood pressure, approximately 10%, with the epidural technique than with the spinal.
[45]
The proposed advantage of slower onset of
epidural
blockade is often mitigated by anesthesiologists administering a decreased volume
of local anesthetic with the initial epidural therapeutic dose; when the block height
does not rise as rapidly as desired, additional epidural local anesthetic is administered,
and a higher block than necessary may result. The extent to which arterial blood
pressure decreases with either technique depends on multiple factors, including patient
age and intravascular volume status.
 |
 Figure 43-6
Anatomic variants of sacrum and sacral hiatus. Sacral
hiatus variants. A, Normal. B,
Longitudinal slitlike hiatus. C, Second midline hiatus.
D, Transverse hiatus. E,
Large hiatus with absent cornua. F, Transverse hiatus
with absent coccyx and two prominent cornua and two proximal "decoy hiatuses lateral
to the cornua." G–I, Large midline defects
contiguous with sacral hiatus. J–L, Enlarged
longitudinal hiatuses, each with an overlying decoy hiatus. (From Willis
RJ: Caudal epidural block. In Cousins MN, Bridenbaugh
PO [eds]: Neural Blockade in Clinical Anesthesia and Management of Pain, 2nd ed.
Philadelphia, JB Lippincott, 1988, p 365.)
Figure 43-6
Anatomic variants of sacrum and sacral hiatus. Sacral
hiatus variants. A, Normal. B,
Longitudinal slitlike hiatus. C, Second midline hiatus.
D, Transverse hiatus. E,
Large hiatus with absent cornua. F, Transverse hiatus
with absent coccyx and two prominent cornua and two proximal "decoy hiatuses lateral
to the cornua." G–I, Large midline defects
contiguous with sacral hiatus. J–L, Enlarged
longitudinal hiatuses, each with an overlying decoy hiatus. (From Willis
RJ: Caudal epidural block. In Cousins MN, Bridenbaugh
PO [eds]: Neural Blockade in Clinical Anesthesia and Management of Pain, 2nd ed.
Philadelphia, JB Lippincott, 1988, p 365.)