|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
After a decision is made to use one of these blocks, the key feature of performing the block is combining appropriate technique with a three-dimensional understanding and tactile appreciation of anatomy.
Subarachnoid local anesthetics affect their sensory block at the spinal cord, which is continuous cephalad with the brainstem through the foramen magnum and terminates distally in the conus medullaris. This distal termination, because of differential growth rates between the bony vertebral canal and central nervous system (CNS), varies from L3 in the infant to the lower border of L1 in the adult. Most of us develop the impression that the spinal nerve roots are uniform in size and structure,
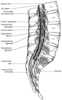 Figure 43-1
Spinal cord anatomy. Notice the termination of the spinal
cord (i.e., conus medullaris) at L1–2 and the termination of the dural sac
at S2 (see Plate 6
in
the color atlas of this volume).
Figure 43-1
Spinal cord anatomy. Notice the termination of the spinal
cord (i.e., conus medullaris) at L1–2 and the termination of the dural sac
at S2 (see Plate 6
in
the color atlas of this volume).
Surrounding the spinal cord in the bony vertebral column are three membranes (from within to the periphery): the pia mater, arachnoid mater, and dura mater ( Fig. 43-1 ; see Plate 6 in the color atlas of this volume). The pia mater is a highly vascular membrane that closely invests the spinal cord and brain. The arachnoid mater is a delicate, nonvascular membrane closely attached to the outermost layer, the dura. Of these two membranes, it is estimated that the arachnoid functions as the principal barrier to drugs crossing in and out of the CSF, accounting for 90% of the resistance to drug migration.[19] As Lui and McDonald[16] emphasize, the functional proof of the arachnoid's role as the primary barrier to flow is the observation that the spinal CSF resides in the subarachnoid and not the subdural space.
In the subarachnoid space are the CSF, spinal nerves, a trabecular network between the two membranes, and blood vessels that supply the spinal cord and the lateral extensions of the pia mater and the dentate ligaments, which supply lateral support from the spinal cord to the dura mater ( Fig. 43-2 ; see Plate 6 in the color atlas of this volume).[20] Although the spinal cord ends at the lower border of L1 in adults, the subarachnoid space continues to S2.
The third and outermost membrane in the spinal canal is the randomly organized fibroelastic membrane, the dura mater (or theca).[21] This layer is the direct extension of the cranial dura mater and extends as spinal dura mater from the foramen magnum to S2, where the filum terminale (an extension of the pia mater beginning at the conus medullaris) blends with the periosteum on the coccyx (see Fig. 43-1 and Plate 6 in the color atlas of this volume). There is a potential space between the dura mater and the arachnoid, the subdural space, which contains only small amounts of serous fluid allowing the dura and arachnoid to move over each other. This space is not intentionally used by anesthesiologists, although injection into it
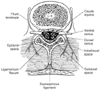 Figure 43-2
Contents of dural sac at the level of L4. The cauda
equina is contained within a dural sac filled with cerebrospinal fluid (see Plate
6
in the color atlas of this volume).
Figure 43-2
Contents of dural sac at the level of L4. The cauda
equina is contained within a dural sac filled with cerebrospinal fluid (see Plate
6
in the color atlas of this volume).
Surrounding the dura mater is another space that is often used by anesthesiologists, the epidural space. The spinal epidural space extends from the foramen magnum to the sacral hiatus and surrounds the dura mater anteriorly, laterally, and more usefully, posteriorly. The epidural space is bounded anteriorly by the posterior longitudinal ligaments, laterally by the pedicles and the intervertebral foramina, and posteriorly by the ligamentum flavum. Contents of the epidural space include the nerve roots that traverse it from foramina to peripheral locations, as well as fat, areolar tissue, lymphatics, and blood vessels, which include the well-organized Batson venous plexus. Hogan[22] suggests from his study of frozen cryomicrotome cadaver sections that the epidural space is more segmented and less uniform than previously believed from indirect anatomic analysis ( Fig. 43-3 ). This lack of epidural space uniformity also extends to age-related differences. There is evidence that adipose tissue in the epidural space diminishes with age.[23] Another anatomic change in epidural space anatomy that has long been promoted is that intervertebral foramina decrease in size with increasing age. This decrease has been linked conceptually to higher block levels for similar epidural doses of local anesthetic. Saitoh and coworkers [24] showed that this concept probably is wrong; they showed no correlation between leakage of radiocontrast through intervertebral foramina and age. When the data of Igarashi and associates[23] and Saitoh and colleagues[24] are considered
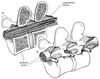 Figure 43-3
A, Sagittal section demonstrates
that the contents of the epidural space depend on level of section. B,
Three-dimensional drawing of the epidural space shows the discontinuity of the epidural
contents, but this potential space can be dilated by injection of fluid into the
epidural space.
Figure 43-3
A, Sagittal section demonstrates
that the contents of the epidural space depend on level of section. B,
Three-dimensional drawing of the epidural space shows the discontinuity of the epidural
contents, but this potential space can be dilated by injection of fluid into the
epidural space.
Posterior to the epidural space is the ligamentum flavum (the so-called yellow ligament), which also extends from the foramen magnum to the sacral hiatus. Although classically portrayed as a single ligament, it is really composed of two ligamenta flava, the right and the left, which join in the middle, forming an acute angle with a ventral opening ( Fig. 43-4 ; Plate 8 in the color atlas of this volume).[22] [25] The ligamentum flavum is not uniform from skull to sacrum, nor even within an intervertebral space. The ligament thickness, distance to dura, and skin-to-dura distance vary with the area of vertebral canal ( Table 43-1 ). The two ligamenta flava are variably joined (fused) in the midline, and this fusion or lack of fusion of the ligamenta flava even occurs at different vertebral levels in individual patients.[22] Immediately posterior to the ligamentum flavum are the lamina and spinous processes of vertebral bodies or the interspinous ligaments. Extending from the external occipital protuberance to the coccyx posterior to these structures is the supraspinous ligament, which joins the vertebral spines (see Fig. 43-4 ).
Occasionally, clinically unilateral anesthesia may follow apparently adequate epidural technique.[26] [27] By means of epiduroscopy, Blomberg[28] identified the universal appearance of a dorsomedian band in the midline of the epidural space. Anatomic dissection and computed tomographic epidurography have also suggested epidural space septa.[29] Hogan and Toth[15] suggest that these anatomic findings really identify an artifact of the midline posterior
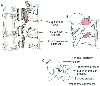 Figure 43-4
Vertebral anatomy. A,
Sagittal view. B, Oblique view of the lumbar vertebrae,
showing ligamentum flavum thickening in the caudad extent of intervertebral space
and in the midline. C, Oblique view of single lumbar
vertebra (see Plate 8
in the color atlas of this volume).
Figure 43-4
Vertebral anatomy. A,
Sagittal view. B, Oblique view of the lumbar vertebrae,
showing ligamentum flavum thickening in the caudad extent of intervertebral space
and in the midline. C, Oblique view of single lumbar
vertebra (see Plate 8
in the color atlas of this volume).
Blomberg[28]
used fiberoptic
technique to demonstrate that the subdural extra-arachnoid space is easily entered
in 66% of autopsy attempts in humans. Despite this being an infrequent clinical
problem with epidural anesthesia, it does allow a visual understanding of subdural
complications of epidural anesthesia.[31]
Blomberg
[28]
continued his
| Site | Skin to Ligament (cm) | Thickness of Ligament (mm) |
|---|---|---|
| Cervical | — | 1.5–3.0 |
| Thoracic | — | 3.0–5.0 |
| Lumbar | 3.0–8.0 * | 5.0–6.0 † |
| Caudal | Variable | 2.0–6.0 |
| Data from Cousins MJ, Bromage PR: Epidural neural blockade. In Cousins MJ, Bridenbaugh PO (eds): Neural Blockade in Clinical Anesthesia and Management of Pain. Philadelphia, JB Lippincott, 1988, p 255 and other sources. | ||
The performance of caudal anesthesia calls for an expanded understanding of epidural anatomy and especially of the frequent variations in sacral anatomy. [33] The sacrum results from the fusion of the five sacral vertebrae. The sacral hiatus, which is the failure of the laminae of S5 and usually part of S4 to fuse in the midline, is the detail of interest. This results in a variably shaped and sized inverted-V-shaped bony defect, covered by the posterior sacrococcygeal ligament, a functional counterpart to the ligamentum flava. This sacral hiatus may be identified by locating the sacral cornua, the remnants of the S5 articular processes ( Fig. 43-5 ). This bony defect allows access to the sacral canal, although needle insertion through this defect may be difficult because of the frequency of anatomic variation. For example, the shape of the space may vary from slitlike to a wide-based, inverted V, and in 1 of 20 patients, the bony defect may be absent, precluding the caudal approach ( Fig. 43-6 ).[34] [35]
The sacral canal contains the terminal portion of the dural sac, which typically ends cephalad to a line joining the posterior superior iliac spines, or S2. Variation is found in this feature as well, with the termination of the dural sac being lower in children, although the ease of palpating the sacral hiatus in children may make pediatric caudal technique easier overall. In addition to the dural sac, the sacral canal contains a venous plexus,
 Figure 43-5
Sacral surface anatomy. An equilateral triangle can
be drawn to connect the posterior superior iliac spines and the sacral hiatus. This
can be useful in confirming palpation of the sacral hiatus.
Figure 43-5
Sacral surface anatomy. An equilateral triangle can
be drawn to connect the posterior superior iliac spines and the sacral hiatus. This
can be useful in confirming palpation of the sacral hiatus.
After the anatomy pertinent to neuraxial blocks is understood, it is tempting to administer the blocks immediately. Nevertheless, safe conduct of spinal, epidural, and caudal anesthesia requires an appreciation for the physiologic effects of these blocks if they are to be used appropriately.
|
|
|
|
|
|
|
|
|
|
|
|
|