|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In addition to the acid-base disturbances seen in emergency situations, a number of acid-base conundrums are peculiar to the perioperative and critically ill patient. These include respiratory acidosis and alkalosis associated with mechanical ventilation, acidosis due to hyperchloremia or dilution, and alkalosis due to sodium gain, chloride loss or volume contraction.
Perioperatively, respiratory acidosis is caused by poor mechanical ventilation strategy, narcosis, or incomplete reversal of neuromuscular blockade. Respiratory alkalosis is caused by hyperventilation in response to pain or anxiety.
Metabolic acid-base disturbances are relatively common perioperatively. Pathologic causes involve lactic acidosis, ketoacidosis, and renal acidosis as described in the previous section. Iatrogenic causes include manipulation of the SID by administration of electrolyte- or osmoliteimbalanced solutions.
Hyperchloremic acidosis is frequently seen in the operating suite. It usually follows large-volume administration of 0.9% saline solution. This solution contains 154 mEq of both Na+ and Cl+ , with an SID of 0. Each liter of normal saline is associated with a decline in the SID value. Hyperchloremic acidosis is also associated with acute normovolemic hemodilution with 5% albumin solution or 6% hetastarch (both formulated in normal saline).[33] Kellum,[34] in an experimental model of sepsis, showed that dogs treated with lactated Ringer's solution and 5% hydroxyethyl starch diluted in lactated Ringer's solution (Hextend) (both with an SID of 20) had less acidosis and longer survival than those treated with normal saline.
There has been an unfortunate tendency to confuse hyperchloremic and dilutional acidosis.[35] Hyperchloremic acidosis is caused by chloride gain, such as by NaCl administration; serum sodium may stay the same or even increase. Dilutional acidosis refers to a condition in which there is an alteration in the relative quantity of sodium and chloride in free water; serum sodium decreases. This can occur perioperatively from administration of sodium-poor fluids. The perioperative stress response is associated with reduced ability to excrete free water because of increased secretion of antidiuretic hormone. Excessive administration of hypotonic fluids, such as 5% dextrose
Traditional teaching explains dilutional acidosis in terms of dilution of bicarbonate by the added volume.[35] However, acids and bases alike should be diluted in this fashion. Moreover, no study has been able to quantitatively explain dilutional acidosis on the basis of reduced serum bicarbonate.[35] [36] Bicarbonate concentration is determined by carbon dioxide equilibrium.[37] Alterations in pH associated with changes in the relative ratio of sodium and chloride have been elegantly demonstrated by Morgan and colleagues.[38] With in vitro hemodilution, there was a simple linear relationship between diluent crystalloid SID and the rate and direction of change of plasma SID and whole blood BE.
What is the relevance of hyperchloremic acidosis? Waters and colleagues[39] demonstrated a significant difference in perioperative acidosis in patients undergoing aneurysm surgery who were treated with normal saline rather than lactated Ringer's solution. There were, however, no significant differences in outcomes. Brill and colleagues[13] found that acidosis due to hyperchloremia was associated with better outcomes than those associated with lactic acidosis or ketoacidosis. This supports the contention that it is the underlying problem that increases the patient's risk, not the acidosis itself.
Hyperchloremic acidosis is treated by increasing the SID of infused fluids, such as by infusing sodium without chloride. Although no such fluid is available commercially, one can be easily made up by diluting 3 ampules of 7.5% sodium bicarbonate in 1 L of 5% dextrose. This is
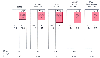 Figure 41-6
Changes in acid-based and electrolyte composition in
patients with respiratory acidosis. Left to right,
the panels depict normal acid-base status; adaptation to an acute rise in the partial
pressure of arterial carbon dioxide (PaCO2
)
to 80 mm Hg; adaptation to a long-term rise in PaCO2
to 80 mm Hg; superimposition of an acute further increment in PaCO2
(to a level of 100 mm Hg) in the same patient; and post-hypercapnic alkalosis resulting
from an abrupt reduction in PaCO2
to the
level of 40 mm Hg in the same patient. A-
denotes unmeasured plasma anions,
and the numbers within the bars give ion concentrations in millimoles per liter.
(Adapted from Androgue H, Madias N: Management of life-threatening acid-base
disorders. First of two parts. N Engl J Med 338:26–34, 1998.)
Figure 41-6
Changes in acid-based and electrolyte composition in
patients with respiratory acidosis. Left to right,
the panels depict normal acid-base status; adaptation to an acute rise in the partial
pressure of arterial carbon dioxide (PaCO2
)
to 80 mm Hg; adaptation to a long-term rise in PaCO2
to 80 mm Hg; superimposition of an acute further increment in PaCO2
(to a level of 100 mm Hg) in the same patient; and post-hypercapnic alkalosis resulting
from an abrupt reduction in PaCO2
to the
level of 40 mm Hg in the same patient. A-
denotes unmeasured plasma anions,
and the numbers within the bars give ion concentrations in millimoles per liter.
(Adapted from Androgue H, Madias N: Management of life-threatening acid-base
disorders. First of two parts. N Engl J Med 338:26–34, 1998.)
Perioperative metabolic alkalosis is usually of iatrogenic origin. Hyperventilation of patients with chronic respiratory failure results in acute metabolic alkalosis because of the chronic compensatory alkalosis associated with chloride loss in urine[8] ( Fig. 41-6 and Table 41-5 ). More frequently, metabolic alkalosis is associated with increased SID due to sodium gain. This is caused by administration of fluids in which sodium is "buffered" by weak ions, citrate (in blood products), acetate (in parenteral nutrition), and bicarbonate. Buffer ions such as citrate, acetate, gluconate, and lactate are, under normal conditions, rapidly cleared by the liver and do not contribute to acid-base balance. Sodium and chloride obey the law of conservation of mass. Sodium gain is chloride-sensitive alkalosis, treated by administration of net loads of chloride in the form of 0.9% NaCl, potassium chloride, calcium chloride, and occasionally, hydrogen chloride. It is important to correct chloride-sensitive alkalosis, because the normal compensatory measure is hypoventilation, which increases PaCO2 and may lead to CO2 narcosis or failure to liberate the patient from mechanical ventilation.
Another cause of metabolic alkalosis in perioperative patients is chloride loss caused by removal of chloride from the gastrointestinal tract by continuous suctioning or vomiting. Gastric juice contains hydrochloric acid, and loss of chloride leads to alkalosis. Loss of hydrogen ions does not.
Anesthesiologists should understand the effects of fluids on acid-base disturbances (see Chapter 46 ). Hypotonic and
| Disorder | Case |
|---|---|
| Respiratory acidosis | Hypoventilation; narcosis, incomplete reversal of neuromuscular blockade |
| Respiratory alkalosis | Hyperventilation; anxiety, pain |
| Metabolic acidosis due to unmeasured anions (e.g., widened gap acidosis) | Hypoperfusion (e.g., lactic acidosis; diabetic ketoacidosis); renal failure |
| Metabolic acidosis due to measured anions (non-anion gap hyperchloremic acidosis) | Hyperchloremia (e.g., "normal" saline, hetastarch, albumin infusions); renal tubular acidosis; bladder reconstructions |
| Metabolic acidosis due to free water excess (e.g., hyponatremia, dilution acidosis) | Hypotonic fluid administration; sodium loss (e.g., diarrhea); administration of hyperosmolar fluids (e.g., mannitol, alcohol); hyperproteinemia |
| Metabolic alkaloiss | Hyperventilation of patient with history of carbon dioxide retention (e.g., chronic obstructive pulmonary disease); sodium gain (e.g., sodium bicarbonate, massive blood transfusion); chloride loss (e.g., nasogastric suctioning) |
|
|
|
|
|
|
|
|
|
|
|
|
|