|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Electroencephalographic signals provide information about cortical
function but little or no information about neural pathways important to the daily
function of
| Cerebral unresponsiveness |
| No spontaneous motor activity |
| Absent pupillary, corneal, and oculocephalic or oculovestibular reflexes |
| Absent cough reflex with deep tracheal suctioning |
| No increase in heart rate in response to intravenous administration of atropine (2 mg) |
| No respiratory efforts on apnea testing (PaCO2 > 60 mm Hg) |
| Electrocerebral silence documented by electroencephalography in the absence of drug effect (desirable) |
| From Darby JM, Stein K, Grenvik A, et al: Approach to management of the heart beating "brain dead" organ donor. JAMA 261:2222, 1989. |
SERs are electrical CNS responses to electrical, auditory, or visual stimuli. SERs are produced by stimulating a sensory system and recording the resulting electrical responses at various sites along the sensory pathway up to and including the cerebral cortex. Because of the very-low-amplitude of SERs (0.1 to 10 µV), it is often not possible to distinguish SERs from other background biologic signals such as those on the EEG or EMG that may be considered in this case undesirable noise. To extract the SER from the background noise, the recorded signal is digitized, and signal averaging is applied. With this technique, signal recording is time-locked to the application of the sensory stimulus. For example, during intraoperative SER monitoring of the median nerve, only signal information occurring less than 50 msec after the stimulus is recorded after nerve stimulation at the wrist. The SER occurs at a constant time after the stimulus application; other electrical activity such as spontaneous electroencephalographic activity occurs at random intervals after the sensory stimulus. The averaging technique improves the SER signal-to-noise ratio by eliminating random elements and enhancing the SER. This enhancing effect increases directly with the square root of the number of responses added into the averaged response.
SER recordings are of two general types determined by the distance of the recording electrode from the neural generator of the evoked response. SERs recorded from electrodes close to the neural generators (within approximately 3 to 4 cm in the average adult) are called near-field potentials. [57] Near-field potentials are transmitted to the recording electrode by propagated conduction along a discrete neural pathway,[58] and the morphology is directly affected by electrode location.[57] Far-field potentials are recorded from electrodes located a greater distance from the neural generator and are conducted to the recording electrode through a volume conductor (i.e., brain, cerebrospinal fluid, and membranes). It is more difficult to locate the source of the recorded signal because the current spreads diffusely throughout the conducting medium, and the electrode position has little effect on the morphology of the recorded evoked potential.[57] [58] As the distance between the recording electrode and the neural generator increases, the recorded SER becomes smaller. More responses have to be averaged to record far-field potentials (up to several thousand) than near-field potentials (as few as 50 to 100).[57] [58]
SERs may also be described as cortical or subcortical in origin. Cortical responses are generated by a summation of excitatory and inhibitory postsynaptic potentials produced by neurons in the gray matter. Subcortical responses may arise from many different structures, depending on the type of response, including peripheral nerves, spinal cord,
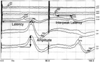 Figure 38-9
Sensory evoked responses are described in terms of latency
(upper left) and amplitude (lower
left). Interpeak latency is the measured time between two peaks. Interpeak
latency may be measured between two peaks in the same channel or between peaks in
different channels (upper right).
Figure 38-9
Sensory evoked responses are described in terms of latency
(upper left) and amplitude (lower
left). Interpeak latency is the measured time between two peaks. Interpeak
latency may be measured between two peaks in the same channel or between peaks in
different channels (upper right).
SERs are described in terms of latency and amplitude ( Fig. 38-9 ). Latency is defined as the time measured from the application of the stimulus to the onset or peak (depending on convention used) of the SER. The amplitude is the voltage of the recorded response. According to convention, deflections below the baseline are labeled positive (P), and those above the baseline are negative (N). Standard identification of waveforms is by letter
 Figure 38-10
Short-latency somatosensory evoked potentials are produced
by stimulation of the median nerve at the wrist. The ability to identify each of
the labeled peaks shown in the tracing from the awake patient is compromised by the
anesthetic state and use of different recording electrode locations. Corresponding
tracings are labeled with the same letter. (Adapted from Chiappa KH, Ropper
AH: Evoked potentials in clinical medicine. N Engl J Med 306:1205, 1982.)
Figure 38-10
Short-latency somatosensory evoked potentials are produced
by stimulation of the median nerve at the wrist. The ability to identify each of
the labeled peaks shown in the tracing from the awake patient is compromised by the
anesthetic state and use of different recording electrode locations. Corresponding
tracings are labeled with the same letter. (Adapted from Chiappa KH, Ropper
AH: Evoked potentials in clinical medicine. N Engl J Med 306:1205, 1982.)
Intraoperative changes in SERs, such as decreased amplitude, increased latency, or complete loss of the waveform, may result from surgical trespass or ischemia, or they may reflect systemic changes such as changes in anesthetic drug administration, temperature changes, or hypoperfusion. When these changes are detected and considered to be significant, the anesthesiologist or surgeon can make changes to relieve or lessen the insult to the monitored pathway. Interventions by the anesthesiologist are directed at improving perfusion to the nervous tissue at risk and include increasing arterial blood pressure, especially if induced hypotension is used or if the patient's pressure has fallen to below the preoperative level; transfusion if significant anemia is present; volume expansion; augmentation of cardiac output; and normalization of arterial blood gas tensions if indicated. Alterations in SERs may warn the surgeon of direct injury to nervous tissue in the operative field. For example, changes in SERs after retractor placement during a craniotomy or after compression of the blood supply to the spinal cord from spinal column distraction promptly allow the surgeon and the anesthesiologist to make appropriate changes to the operative procedure and anesthetic management that may prevent or minimize any postoperative neurologic deficit.
The degree and duration of SER changes that can be tolerated by patients during surgery is not well known or studied. Tolerance limits for the degree of change in SER signals or duration of complete loss of waveform before permanent neurologic dysfunction occurs are not clearly defined. Such ambiguity, unfortunately, is all too common among intraoperative monitors. For example, although we do know that increased frequency and duration of ST-depression during coronary bypass surgery is associated with an increased risk of perioperative infarction, exact limits for the degree and duration of ST-segment depression for surgery do not exist and probably vary significantly from patient to patient. Many centers using intraoperative SER monitoring define decreases in amplitude of 50% or more from baseline associated with a more than 10% prolongation in latency as clinically significant SER changes. Uncorrected, such changes are associated in clinical series and in case reports with onset of new postoperative neurologic deficits. As a result, such changes are immediately investigated. In practice, however, any SER changes directly associated with a surgical event are considered clinically significant, even if the magnitude of change is less than described earlier. Changes in SER that do not progress to complete loss of the waveform are less likely to be associated with a major new postoperative neurologic deficit. Complete loss of the SER waveform intraoperatively without recovery is highly likely to be associated with a major new deficit. If the SER recovers spontaneously or after intraoperative interventions, the likelihood of neurologic injury depends on the procedure and the duration of the SER loss. For example, in one study of aortic vascular surgery where SSEPs were monitored, loss of the SSEP waveform for less than 15 minutes was not associated with a new permanent neurologic deficit, whereas complete loss of the SSEP for longer periods was increasingly likely to reflect permanent neurologic injury, even if the response recovered completely to normal during surgery.[59]
The operating room is a hostile environment for recording SERs. Because these potentials have very small amplitudes, the electrical noise that is quite common in most operating rooms often interferes with recording of good-quality SERs. Biologic noise, such as the cardiac electrical activity (i.e., electrocardiographic activity) and muscle movement or increased tone (i.e., electromyographic activity), may obscure the desired evoked response. To successfully record SERs in the operating room environment, these sources of noise must be recognized and the appropriate action taken to allow recording of the desired evoked response (e.g., unnecessary electrical equipment unplugged, small doses of muscle relaxant given to minimize electromyographic activity). Several physiologic changes and drugs administered intraoperatively can result in changes in the SERs unrelated to potential injury to nervous tissue. These factors must be recognized, minimized if possible, and included in the differential diagnosis as changes in SERs are detected intraoperatively. One of the most important principles of recording SERs intraoperatively is that reproducible, reliable tracings must be obtained at baseline before any intervention likely to cause changes in the evoked response. If good-quality tracings with identifiable waveforms cannot be recorded and reproduced at baseline, SER monitoring will be of little use in monitoring the integrity of the CNS intraoperatively. If significant variability exists or waveforms are difficult to identify, it will not be possible intraoperatively to distinguish SER changes that are clinically significant from a preexisting baseline variability of waveforms. When good, reproducible responses cannot be recorded at baseline, monitoring should not be used for clinical decision-making.
SSEPs are recorded after electrical stimulation of a peripheral mixed nerve. Stimulation is provided most commonly with surface electrodes (e.g., electroencephalographic electrodes) placed on the skin above the nerve or with fine-needle electrodes. A square wave stimulus of 50 to 250 µsec duration is delivered to the peripheral nerve, and the intensity is adjusted to produce a minimal muscle contraction. Increasing the stimulus intensity beyond the sum of the motor and sensory threshold does not influence the amplitude or latency of the recorded evoked potential. [55] On a practical basis, however, many laboratories do not establish SSEP monitoring until after the patient is already anesthetized and paralyzed. In these cases, stimulus intensity is increased until no further increase in response size occurs at any recording site, and constant current stimulation of 20 to 50 mA is quite commonly used. For comparison, consider supramaximal stimulation used for neuromuscular blockade monitoring, commonly 80 mA. The rate of stimulation varies from 1 to 6 Hz. The common sites of stimulation include median nerve at the wrist, common peroneal nerve at the knee, and posterior tibial nerve at the ankle. [60] The tongue, trigeminal nerve, pudendal nerve, and ulnar nerve have
SSEP responses consist of short- and long-latency waveforms. Short-latency SSEPs are most commonly recorded intraoperatively, because they are less influenced by changes in anesthetic drug levels. The pathways involved in the generation of upper extremity short-latency SSEPs include large-fiber sensory nerves with their cell bodies in the dorsal root ganglia and central processes traveling rostrally in the ipsilateral posterior column of the spinal cord and synapsing in the dorsal column nuclei at the cervicomedullary junction (i.e., first-order fibers), second-order fibers crossing and traveling to the contralateral thalamus through the medial lemniscus, and third-order fibers from the thalamus to the frontoparietal sensorimotor cortex.[55] These primary cortical evoked responses, which are recordable with most anesthetic techniques, result from the earliest electrical activity generated by the cortical neurons and are thought to arise from the postcentral sulcus parietal neurons. The longer-latency secondary cortical responses are thought to arise in the association cortex. These responses have much greater variability in the awake patient,[58] habituate rapidly on repetitive stimulation,[57] and are not reproducible during general anesthesia. Cortical SSEPs other than the primary cortical response are not monitored or interpreted intraoperatively because they are severely altered by general anesthesia.[57]
Whereas most evidence indicates that upper extremity evoked potentials are conducted rostrally in the spinal cord through dorsal column pathways, many data suggest that lower extremity SSEPs are conducted substantially by the lateral funiculus. [61] Stimulation of the posterior tibial nerve or common peroneal nerve at or above motor threshold activates group I fibers that synapse and travel rostrally through the dorsal spinocerebellar tract. After synapsing in nucleus Z at the spinomedullary junction, the pathway crosses and projects onto the ventral posterolateral thalamic nucleus.[62] Studies with dogs, cats, and monkeys support the concept that lower extremity SERs are conducted in all quadrants of the spinal cord but primarily in the dorsal lateral funiculus. [63] [64] This pathway difference is important because the dorsal lateral funiculus is supplied primarily by the anterior spinal artery, the artery that also supplies the descending motor pathway and neurons in the spinal cord. Manipulations such as distraction of the spinal column to correct scoliosis, which may secondarily compress or distort radicular blood supply to the anterior spinal cord, should cause changes in the SSEP in the event blood supply is reduced to critical levels. This hypothesis is verified by the very low, but not zero, incidence of postoperative paraplegia on awakening without any intraoperative changes in SSEP.
For SSEP recordings with median nerve stimulation, recording electrodes (i.e., usually electrocardiographic pads) are first placed at Erb's point, just above the midpoint of the clavicle. This point overlies the brachial plexus, and signals recorded here assure the clinician that the stimulus is being delivered properly to the patient. The next electrode (i.e., an electrocardiographic pad or a gold cup electrode) is placed midline posteriorly over the neck at level of the second cervical vertebra, relatively near the dorsal column nuclei. Signals recorded here ensure proper transmission of the response from the peripheral nervous system into the spinal cord and rostral along the spinal cord to the lower medulla. The final electrodes (i.e., gold cup electrodes or needle electrodes) are placed on the scalp overlying the sensory (parietal) cortex contralateral to the stimulated limb. Signals recorded here ensure the integrity of the pathway through the brainstem, thalamus, and internal capsule and may also assess adequacy of CBF in this area of the cortex. [65] [66] [67] [68] [69] For recording SSEPs after posterior tibial nerve stimulation, electrodes (i.e., electrocardiographic pads) are placed first over the politeal fossa to ensure proper stimulus delivery to the nervous system. Electrodes may also be placed over the lower lumbar spine to ensure proper transmission of the signal into the spinal cord itself, but this site is not commonly used because of the proximity of sterile surgical incisions. Cervical spine and scalp recording electrodes are placed in a fashion similar to that described earlier, although different locations may be used as required by the placement of the surgical incision.
More invasive recording methods may also be used intraoperatively. These methods, although introducing an element of risk to the patient, have the advantage of producing much larger signals. For example, cortical SSEPs may be recorded from electrodes placed directly on the cerebral cortex, and subcortical potentials may be recorded using electrodes placed invasively into bone, ligament, or the epidural space in or near the operative site.[57]
Purported neural generators for short-latency SSEPs are listed
in Table 38-7
and Figure
38-10
.[57]
[60]
Induction of anesthesia and use of different recording electrode locations (i.e.,
montage), necessitated by the surgical incision, may significantly alter the appearance
of the SSEP. In these cases, attribution of a particular generator to a given wave
on the tracing may be quite difficult. During neurologic monitoring, such precision
is not needed, and recorded waveforms are compared with tracings obtained at baseline
and during earlier portions of the surgical procedure. After lower limb stimulation,
absolute latencies are increased because of the greater distance the response to
stimulation must travel along the peripheral sensory nerve and spinal cord. Interpeak
latencies (see Fig. 38-9
)
| Peak Waveforms | Neural Generators |
|---|---|
| N9 (EP) | Brachial plexus * |
| N11 | Posterior columns or spinal roots |
| N13/P13 | Dorsal column nuclei * |
| N14,15 | Brainstem or thalamus |
| N19/P22 | Parietal sensory cortex * |
| EP, Erb point; N, indicates negative for the number of milliseconds between the stimulus and response; P, indicates positive for the number of milliseconds between the stimulus and response. | |
For SSEP monitoring to benefit a patient, the neurologic pathway monitored must be potentially at risk during the procedure, and if SSEP changes occur, an option for intervention must exist. If both criteria are not true, monitoring is likely to offer little benefit to the patient. To optimize conditions for SSEP monitoring, the anesthesiologist should choose an anesthetic technique that does not markedly depress the SSEP, and the anesthetic depth and physiologic state of the patient should remain as constant as possible during periods of potential surgical injury to the monitored pathway. Reliable baseline tracings should be obtained before any intervention.
Intraoperative recording of SSEPs has been used to assess the functional integrity of the sensory pathways during operative procedures that place these pathways at risk as a result of surgical trespass along the course of the peripheral nerve, within the spinal canal, or in the brain or when there is potential for damage to the sensory pathway or compromise of its vascular supply. SSEP monitoring has also been used to assess the integrity of anatomically adjacent structures that are more difficult to monitor directly, such as the motor tracts. Intraoperative SSEP monitoring has been described for a wide variety of procedures, including correction of scoliosis with instrumentation[71] [72] ; spinal cord decompression and stabilization after acute spinal cord injury[73] [74] ; spinal fusion[75] ; brachial plexus exploration after injury to the plexus[76] ; resection of spinal cord tumor, cyst, or vascular lesion[77] ; correction of cervical spondylosis[77] ; resection of fourth ventricular cyst[77] ; release of a tethered cord[77] ; resection of intracranial vascular lesions involving the sensory cortex[77] ; clipping of intracranial aneurysms[59] ; carotid endarterectomy[78] ; resection of thalamic tumor[79] ; surgical correction after thoracic spine fracture[80] ; abdominal and thoracic aortic aneurysm repair[55] ; and repair of coarctation of the aorta.[81]
Two intraoperative uses of SSEPs have been most successful and deserve special mention. Intraoperative monitoring of SSEPs has been used most often in patients undergoing surgical procedures involving the spinal column or spinal cord, or both. Extensive experience has been gained in patients who have decompressive laminectomies or who have undergone corrective procedures for scoliosis. Intraoperative changes in SSEPs have been observed in 2.5% to 65% of patients undergoing surgical procedures on the spine or spinal cord.[72] [75] [80] [82] When these changes are promptly reversed spontaneously or with interventions by the surgeon or anesthesiologist (e.g., lessening the degree of spine straightening in scoliosis surgery, increasing arterial blood pressure), the patients most often have preserved neurologic function postoperatively. When these changes persisted, however, the patients most often awakened with worsened neurologic function.
False-negative (rare) and false-positive (common) results have been reported with SSEP monitoring during spinal surgery. Patients with intact SSEPs throughout the procedure have awakened with a new significant neurologic deficit, but the total reported incidence of this finding is far less than 1% of all cases monitored. However, patients with no postoperative neurologic deficit commonly experience significant (as defined earlier) changes in intraoperative SSEPs.[82] This monitoring pattern is most commonly caused by failure to control for other, non-pathologic factors that may alter the SSEP. Overall, the reliability of properly performed SSEP monitoring to predict the postoperative sensory and motor function has been excellent.[57] [77] [80] However, motor tracts are not directly monitored by SSEPs. The blood supply to the dorsal columns of the spinal cord that carry all of the upper extremity SSEP, and at least a portion of the lower extremity SSEP, is derived primarily from the posterior spinal arteries. The blood supply to motor tracts and neurons is derived primarily from the anterior spinal artery. It is therefore possible for a significant motor deficit to develop postoperatively in patients with intact SSEPs throughout the operative course. Such events have been reported.[73] [83]
In operations on the spinal column and after acute spinal cord injury, the sensory and motor changes generally correlate well[57] ; however, in patients suffering neurologic dysfunction after thoracic aortic vascular surgery, posterior spinal cord function (e.g., proprioception, vibration, light touch) may be left intact when motor and other sensory functions (e.g., pain, temperature) are impaired. This result occurred in 32% of patients with neurologic injury after aortic aneurysm repair in one series,[84] with similar results in many other series. Intraoperative SSEP monitoring in these patients carries a significant risk for false-negative results, and as a result, use of such monitoring has not gained significant popularity.
SSEP monitoring has also been used to gauge the adequacy of CBF to the cerebral cortex and subcortical pathways during carotid vascular surgery[69] [85] [86] (see Chapter 52 ) and during intracranial aneurysm surgery[68] [87] [88] [89] [90] [91] [92] (see Chapter 53 ). SSEPs have been found in the laboratory to have a similar but slightly lower threshold for failure when compared with the EEG. SSEPs are generally intact until the cortical blood flow drops below 15 mL per 100 g of tissue per minute.[67] In a few studies comparing SSEP monitoring with the EEG, a similar sensitivity and specificity for postoperative neurologic function was found. These studies, however, involved few patients and are inadequate to support the hypothesis that SSEP monitoring may substitute for electroencephalographic monitoring of the adequacy of CBF.[85] [93] Logic also suggests that changes in SSEP are unlikely, such as for ischemia involving the anterior portions of the frontal or temporal lobe, which could readily be detected by an appropriately placed electroencephalographic electrode. A meta-analysis does not support the use of SSEPs during carotid vascular surgery as a sole monitor of neurologic function.[94]
The SSEP has been much more extensively studied during cerebral aneurysm surgery. During these procedures, the surgical incision precludes placement of scalp electrodes that could detect cerebral ischemia in at-risk cortex.
SSEPs have been used diagnostically in the intensive care unit setting to evaluate comatose patients and assess prognosis. Prolongation of central conduction in comatose patients has been associated with a worse long-term prognosis. [99] Prolongation of central conduction time in patients after subarachnoid hemorrhage is associated with transient neurologic deficits and precedes the development of these deficits. The changes in central conduction time are probably related to cerebral ischemia.[100] If SSEPs have been irreversibly severely damaged or lost after head injury or global hypoxic or hypotensive cerebral injury, the best outcome that can be expected is a chronic vegetative state. In contrast, several reports suggest that patients with intact SSEPs after similar injury may have an excellent prognosis despite initially grave clinical presentations, and a meta-analysis suggests great utility for SSEPs in predicting which patients have the potential to awaken when performed early after the onset of coma.[101] [102] [103] [104]
|
|
|
|
|
|
|
|
|
|
|
|
|