Effects of Hemodynamic Changes
Prerenal and renal factors can generate ischemic renal dysfunction.
There appears to be an early phase of renal adaptation, or pre-prerenal failure,
to the decreased delivery of blood to the glomerulus.[18]
When these compensatory mechanisms become decompensatory, prerenal failure ensues.
Renal clearance is determined by the delivery of waste products to the kidney (i.e.,
renal blood flow) and by the kidney's ability to extract them (i.e., the GFR).
A variety of experimental models have been devised to simulate
hemodynamically mediated human ARF.[64]
[65]
[66]
In these models, renal blood flow is commonly
interrupted mechanically or pharmacologically. A reduction of flow by more than
50% is the rule during the initial phase of the experiment. After transient but
total renal ischemia for 40 to 60 minutes, a lesion appears with clinical manifestations,
laboratory features, and pathologic features of ARF. During the established phase,
GFR is disproportionately depressed, compared with a moderate decline in renal blood
flow. The maintenance of adequate blood supply to the kidneys therefore is critical;
however, determining what constitutes adequate blood supply (i.e., oxygen) is difficult.
The exact role and the timing of changes in renal blood flow in the initiation and
the maintenance of ARF continue to be studied.
A number of theories have been proposed to explain the pathogenesis
of hemodynamically mediated ARF. Although
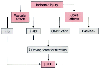 Figure 37-5
Schematic representation of the mechanisms that initiate
and maintain decreased glomerular filtration seen in experimentally induced acute
renal failure. GFR, glomerular filtration rate; Kf
, glomerular capillary
ultrafiltration coefficient; RBF, renal blood flow. (Adapted from Hostetter
TH, Wilkes BM, Brenner BM: Mechanisms of impaired glomerular filtration in acute
renal failure. In Brenner BM, Stein JH [eds]: Acute
Renal Failure. New York, Churchill Livingstone, 1980, p 52.)
Figure 37-5
Schematic representation of the mechanisms that initiate
and maintain decreased glomerular filtration seen in experimentally induced acute
renal failure. GFR, glomerular filtration rate; Kf
, glomerular capillary
ultrafiltration coefficient; RBF, renal blood flow. (Adapted from Hostetter
TH, Wilkes BM, Brenner BM: Mechanisms of impaired glomerular filtration in acute
renal failure. In Brenner BM, Stein JH [eds]: Acute
Renal Failure. New York, Churchill Livingstone, 1980, p 52.)
a reduction in renal blood blow is clearly the initiating cause ( Fig.
37-5
),[67]
[68]
the occurrence of ARF after hypotension is unpredictable. The precise circumstances
precipitating a change from an underperfused healthy kidney to a damaged organ are
difficult to define. Even with decreased delivery of blood to the glomerulus, a
series of compensatory mechanisms may still preserve renal filtration function[18]
( Fig. 37-6
). Salt and water
are retained to restore intravascular volume and fractional tubular reabsorption.
The distribution of cardiac output to the kidneys is also regulated. At a given
level of cardiac output, intrarenal factors affect the ratio of renal-to-systemic
vascular resistance, thereby influencing the percentage of cardiac output received
by the kidneys. Another critical component in the intrinsic modulation of renal
filtration function is the regulation of filtration fraction. At the glomerular
capillary, plasma is separated into a protein-free ultrafiltrate and a nonfiltered
portion. Normally, the filtration fraction (i.e., relationship of GFR to renal plasma
flow) is about 0.2. Initially, the filtration fraction is maintained by efferent
arteriolar constriction. Unabated, the mechanisms that influence efferent arteriolar
vasoconstriction ultimately may influence afferent arteriolar vasoconstriction.
The resulting decrease in filtration fraction is the hallmark of postischemic ARF.
[18]
Ischemic tubular damage may be exacerbated
further by an imbalance between oxygen supply and demand. Most vulnerable to the
imbalance are the thick ascending tubular cells of the loop of Henle in the medulla.
[68]
[69]
[70]
[71]
Intrarenal distribution of blood flow and total renal blood flow
can be assessed with radioactive gases or
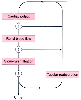 Figure 37-6
The regulatory mechanisms through which glomerular filtration
is modulated include (1) fractional regional blood flow (renal blood flow/cardiac
output); (2) filtration fraction (glomerular filtration rate/glomerular plasma flow
rate); and (3) fractional tubular fluid reabsorption (tubular reabsorption/glomerular
filtration rate). (Adapted from Badr KF, Ichikawa I: Prerenal failure:
A deleterious shift from renal compensation to decompensation. N Engl J Med 319:623,
1988.)
Figure 37-6
The regulatory mechanisms through which glomerular filtration
is modulated include (1) fractional regional blood flow (renal blood flow/cardiac
output); (2) filtration fraction (glomerular filtration rate/glomerular plasma flow
rate); and (3) fractional tubular fluid reabsorption (tubular reabsorption/glomerular
filtration rate). (Adapted from Badr KF, Ichikawa I: Prerenal failure:
A deleterious shift from renal compensation to decompensation. N Engl J Med 319:623,
1988.)
radiolabeled microspheres. Microsphere, xenon washout, and angiographic techniques
have shown that outer cortical blood flow decreases in ischemic models of ARF.[72]
[73]
[74]
Because
85% to 90% of renal blood flow is distributed to cortical glomeruli, the finding
of cortical pallor and redistribution of renal blood flow away from the cortex means
that redistribution may be responsible for the functional lesions of ARF. In some
cases, the return of perfusion to the cortex has correlated with a return of renal
function.[73]
The theory that intrarenal distribution
of blood flow away from the outer cortex to the inner medulla decreases oxygen supply
while increased tubular reabsorption of solute increases oxygen demand is further
supported by studies of renal energetics during ARF.[75]
[76]
[77]
It has
been postulated that a decrease in the GFR and consequently in the energy requirement
for tubular reabsorption may be a mechanism by which the kidney reduces energy demands
before energy supply is critically limited. Increased demand and decreased supply
are precipitated during states of inadequate perfusion, such as those resulting from
depleted intravascular volume, maldistribution of systemic flow away from the renal
vascular bed, cardiogenic shock, and destructive vascular conditions.
 Figure 37-5
Schematic representation of the mechanisms that initiate
and maintain decreased glomerular filtration seen in experimentally induced acute
renal failure. GFR, glomerular filtration rate; Kf
, glomerular capillary
ultrafiltration coefficient; RBF, renal blood flow. (Adapted from Hostetter
TH, Wilkes BM, Brenner BM: Mechanisms of impaired glomerular filtration in acute
renal failure. In Brenner BM, Stein JH [eds]: Acute
Renal Failure. New York, Churchill Livingstone, 1980, p 52.)
Figure 37-5
Schematic representation of the mechanisms that initiate
and maintain decreased glomerular filtration seen in experimentally induced acute
renal failure. GFR, glomerular filtration rate; Kf
, glomerular capillary
ultrafiltration coefficient; RBF, renal blood flow. (Adapted from Hostetter
TH, Wilkes BM, Brenner BM: Mechanisms of impaired glomerular filtration in acute
renal failure. In Brenner BM, Stein JH [eds]: Acute
Renal Failure. New York, Churchill Livingstone, 1980, p 52.) Figure 37-6
The regulatory mechanisms through which glomerular filtration
is modulated include (1) fractional regional blood flow (renal blood flow/cardiac
output); (2) filtration fraction (glomerular filtration rate/glomerular plasma flow
rate); and (3) fractional tubular fluid reabsorption (tubular reabsorption/glomerular
filtration rate). (Adapted from Badr KF, Ichikawa I: Prerenal failure:
A deleterious shift from renal compensation to decompensation. N Engl J Med 319:623,
1988.)
Figure 37-6
The regulatory mechanisms through which glomerular filtration
is modulated include (1) fractional regional blood flow (renal blood flow/cardiac
output); (2) filtration fraction (glomerular filtration rate/glomerular plasma flow
rate); and (3) fractional tubular fluid reabsorption (tubular reabsorption/glomerular
filtration rate). (Adapted from Badr KF, Ichikawa I: Prerenal failure:
A deleterious shift from renal compensation to decompensation. N Engl J Med 319:623,
1988.)