|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The most straightforward method of ascertaining PaO2
is to measure it directly from a blood sample, usually by using a Clark electrode,
which incorporates platinum and reference electrodes in an electrolyte bath (see
Chapter 41
). When a negative
polarizing voltage is applied to the platinum electrode, O2
molecules
in solution are reduced by electrons from the electrode. The electrons from the
cathode react as follows:
O2
+ 2H2
O + 2e−
→ H2
O2
+ 2OH−
H2
O2
+ 2e−
→ 2OH−
(15)
Each molecule of O2 is therefore reduced to two hydroxyl ions by four electrons. In the Clark electrode, the electrolyte solution is separated from the fluid being measured (e.g., blood) by a thin O2 -permeable membrane. The current generated is proportional to PO2 within the sample.
When CO2
is equilibrated with an aqueous solution,
the concentration of carbonic acid is proportional to the PCO2
.
H2
CO3
= α PCO2
(16)
H2
CO3
↔ H+
+
HCO3
−
(17)
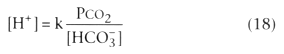
In these equations, α is a constant, and k is a constant that equals 24 if the units are nanomoles/L for [H+ ], mm Hg for PCO2 , and mmol/L for [HCO3 - ]. PCO2 electrodes work by measuring the change in pH induced when blood equilibrates with a potassium chloride-sodium bicarbonate solution.[31]
The history of the development of blood gas analysis has been described by Severinghaus and Astrup[32] and that of O2 monitoring in general by Severinghaus.[33] To obviate the need to withdraw blood samples for PO2 analysis, intra-arterial PO2 monitors have been developed. Initial attempts to use a Clark-type electrode were confounded by problems of drift and blood coagulation. This has been largely replaced by a fiberoptic technique, based on the property of O2 to absorb energy from excited electrons in a fluorescent dye. Incident light is used to elevate electrons in the dye to a higher energy state. These excited electrons may then return to a lower energy level, emitting a photon in the process. Molecular O2 , by absorbing the energy, inhibits the photon emission. This process, called fluorescence quenching, is related to the PO2 . [34]
A system using this technique (Diametrics Medical, St. Paul, MN), which has a ruthenium indicator to measure PO2 , also incorporates an optical absorbance technique to measure PCO2 and pH. Blood pH is measured by continuous
The fiberoptic probes of commercially available systems are somewhat
fragile, and the probes can be subject to movement artifact. However, bias,
the mean difference between instrument measurements and traditional blood gas analysis,
is typically 5%, and imprecision,[35]
the standard deviation of these differences, is about 10%, which are quite acceptable
for clinical purposes. The 90% response time for pH is 70 to 80 seconds and 140
seconds for PO2
and PCO2
;
there is minimal drift over several days.[36]
Use
of continuous arterial blood gas monitoring systems has been described in several
clinical settings[36]
[37]
[38]
[39]
and its
use reviewed.[40]
Umbilical artery insertion of
a probe
| pH |
|
|
|
ΔpH/ΔT = - 0.0146 + 0.0065 (7.4 - pHm ) |
|
|
ΔpH/ΔT = - 0.015 |
|
|
ΔpH/ΔT = - 0.0147 + 0.0065 (7.4 - pHm ) * |
|
|
ΔpH/ΔT = - 0.0146 |
| PCO2 |
|
|
|
Δlog10 PCO2 /ΔT = 0.019 * |
|
|
Δlog10 PCO2 /ΔT = 0.021 |
| PO2 |
|
|
|
|
|
|
Δlog10 PO2 /ΔT = 0.0052 + 0.27 [1 - 10-0.13(100-SaO2 ) ] |
|
|
|
|
|
|
|
|
SO2 ≤ 95%: Δlog10 PCO2 /ΔT = 0.31 |
|
|
SO2 > 95%: Δlog10 PCO2 /ΔT = 0.032 - 0.0268e(0.3SO2 - 30) |
| Hb, blood hemoglobin concentration in g/dL; pHm and PO2m , pH and PO2 values measured at an electrode temperature of 37°C; PO2 , partial pressure of oxygen in mm Hg; SO2 , hemoglobin-oxygen (Hb-O2 ) saturation in percent; T, temperature in degrees centigrade (°C). | |
| Data from Ashwood ER, Kost G, Kenny M: Temperature correction of blood-gas and pH measurements. Clin Chem 29:1877, 1983, and Siggaard-Andersen O, Wimberley PD, Gothgen I, Siggaard-Andersen M: A mathematical model of the hemoglobin-oxygen dissociation curve of human blood and of the oxygen partial pressure as a function of temperature. Clin Chem 30:1646, 1984.) | |
When arterial puncture cannot be achieved or may be technically difficult (e.g., in neonates), capillary PO2 may approximate the arterial value, particularly if the sampling site (e.g., heel) is prewarmed, causing an abundance of local blood flow relative to local tissue O2 consumption. Although capillary PO2 tends to be lower than the arterial value because of the shallow slope of the upper part of the Hb-O2 dissociation curve, capillary O2 saturation approximates arterial O2 saturation (SaO2 ) (capillary value is usually slightly lower).
Conventionally, the temperature of the electrodes in blood gas analyzers is maintained at 37°C, but rarely is a patient's body temperature exactly this temperature (see Chapter 41 ). A blood gas sample from a patient is warmed or cooled to 37°C before analysis. Temperature changes cause alterations in gas solubility in plasma and O2 affinity for hemoglobin. Because solubility and Hb-O2 affinity decrease as temperature rises (and vice versa), if a syringe of blood is gas tight and no bubbles are present, heating the sample will elevate blood gas tensions and reduce the pH; cooling the sample will cause the reverse. Several algorithms exist for temperature correction, allowing the true partial pressure, predictive of physical and chemical activity at the tissue, to be calculated ( Table 36-1 ).
 Figure 36-8
Temperature correction of blood PO2
:
percentage error of PO2
measurement at
various body temperatures with the blood gas electrode at 37°C. If the patient's
temperature is 20°C, a PO2
measurement
performed at 37°C would overestimate the true value by more than 200%. The curves
demonstrate that the percentage of error is greater for venous values (PO2
< 100 mm Hg) than for arterial measurements (PO2
> 300 mm Hg). (Adapted from Camporesi EM, Moon RE: Arterial blood gas
values should be corrected for body temperature during hypothermia. In
Fyman PN, Gotta AW [eds]: Controversies in Cardiovascular Anesthesia. Boston, Kluwer
Academic Publishers, 1988, p 35.)
Figure 36-8
Temperature correction of blood PO2
:
percentage error of PO2
measurement at
various body temperatures with the blood gas electrode at 37°C. If the patient's
temperature is 20°C, a PO2
measurement
performed at 37°C would overestimate the true value by more than 200%. The curves
demonstrate that the percentage of error is greater for venous values (PO2
< 100 mm Hg) than for arterial measurements (PO2
> 300 mm Hg). (Adapted from Camporesi EM, Moon RE: Arterial blood gas
values should be corrected for body temperature during hypothermia. In
Fyman PN, Gotta AW [eds]: Controversies in Cardiovascular Anesthesia. Boston, Kluwer
Academic Publishers, 1988, p 35.)
Application of the equation for PO2 is shown in Figure 36-6 . The PO2 derived from a 37°C electrode is overestimated if the patient is hypothermic and underestimated if the patient is febrile. At high PO2 values (>400 mm Hg) the effect is small, because hemoglobin is fully saturated in this region. At PO2 values below 100 mm Hg, however, the degree of overestimation may be significant. For example, at a patient temperature of 30°C and a PO2 below 80 mm Hg, the true PO2 is overestimated by about 60% unless temperature correction is applied ( Fig. 36-8 ).
Available evidence indicates that, during hypothermia, corrected arterial pH should be maintained in the alkalotic range (i.e., maintain uncorrected pH close to 7.4) (see Chapter 41 ). In contrast, the analysis previously presented suggests that PO2 values should be corrected for temperature.[42]
|
|
|
|
|
|
|
|
|
|
|
|
|