 |
 |
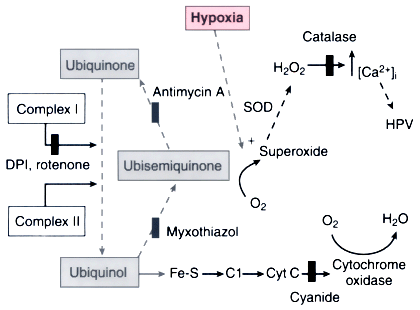
Figure 17-9
Schematic model of the mitochondrial O2
-sensing
and effector mechanism probably responsible for hypoxic pulmonary vasoconstriction
(HPV). In this model, reactive O2
species (ROS) are released from electron
transport chain complex III and act as second messengers in the hypoxia-induced calcium
(Ca2+
) increase and resultant HPV. The solid arrows
represent electron transfer steps; solid bars show
sites of electron chain inhibition. Normal mitochondrial electron transport involves
the movement of reducing equivalents generated in the Krebs cycle through complex
I or II and then through complex III (ubiquinone) and IV (cytochrome oxidase). The
Q cycle converts the dual electron transfer in complex I and II into a single electron
transfer step used in complex IV. The ubisemiquinone (a free radical) created in
this process can generate superoxide, which in the presence of superoxide dismutase
(SOD) produces H2
O2
, the probable mediator of the hypoxia-induced
increased Ca2+
and HPV. This process is amplified during hypoxia. (DPI,
diphenyleneiodonium. DPI, rotenone, and myxothiazol [not shown in figure] are inhibitors
of the proximal portion of the electron transport chain.) (From Waypa GB,
Marks JD, Mack MM, et al: Mitochondrial reactive oxygen species trigger calcium
increases during hypoxia in pulmonary artery myocytes. Circ Res 91:719–726,
2002.)
 |
