 |
 |
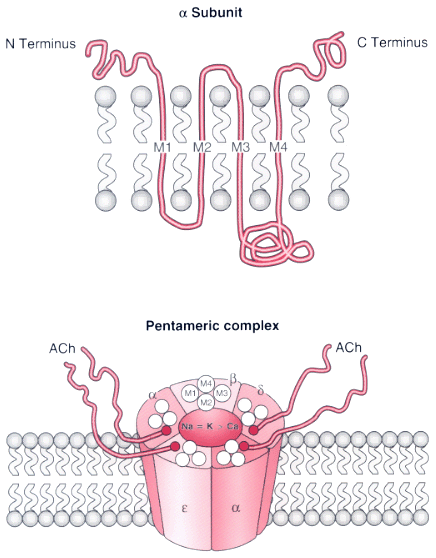
Figure 13-1
Subunit composition of the nicotinic acetylcholine receptor
(nAChR) in the end-plate surface of adult mammalian muscle. The adult AChR is an
intrinsic membrane protein with five distinct subunits (α2
βδepsilon).
Each subunit contains four helical domains labeled M1 to M4. The M2 domain forms
the channel pore. The upper panel shows a single
α-subunit with its N and C termini on the extracellular surface of the membrane
lipid bilayer. Between the N and C termini, the α-subunit forms four helices
(M1, M2, M3, and M4) that span the membrane bilayer. The lower
panel shows the pentameric structure of the nAChR of adult mammalian muscle.
The N termini of two subunits cooperate to form two distinct binding pockets for
acetylcholine (ACh). These pockets occur at the epsilon-α and the δ-α
subunit interface. The M2 membrane-spanning domain of each subunit lines the ion
channel. The doubly liganded ion channel has permeability equal to that of Na+
and K+
; Ca2+
contributes approximately 2.5% to the total permeability.
(Redrawn from Naguib M, Flood P, McArdle JJ, et al: Advances in neurobiology
of the neuromuscular junction: Implications for the anesthesiologist. Anesthesiology
96:202–231, 2002.)
 |
