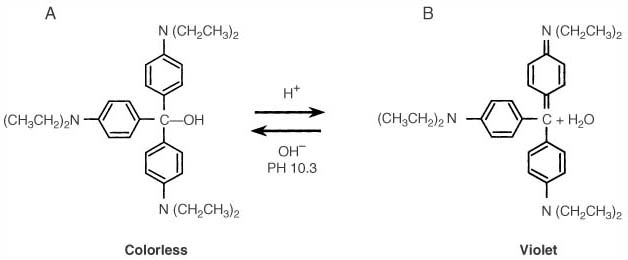
Figure 9-23
Ethyl violet changes from colorless to violet when the
pH of the absorbent decreases as a result of carbon dioxide absorption. A,
The pH of fresh absorbent exceeds the critical level, and the dye exists in its colorless
form. B, As absorbent becomes exhausted, the pH decreases
below 10.3, and ethyl violet changes to its violet form through alcohol dehydration.
(Adapted from Andrews JJ, Johnston RV Jr, Bee DE, Arens JF: Photodeactivation
of ethyl violet: A potential hazard of Sodasorb. Anesthesiology 72:59, 1990.)