|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The sine qua non of the anesthetized state is unconsciousness, the lack of conscious processing of thoughts. The crux of the difficulty in defining "anesthetic depth" is that unconsciousness cannot be measured directly. What can be measured is response to stimulation. Does the patient move when his name is called? Does the response to incision suggest conscious perception? Does the heart rate or blood pressure go up in response to surgical manipulation? Does the patient remember events, conversations, or pain? As observed by Prys-Roberts (see Fig. 31-2 ), anesthesia is nonresponsiveness, broadly defined. The "depth" of anesthesia is determined by the stimulus applied, the response measured, and the drug concentration at the site of action that blunts responsiveness.
As defined elsewhere in this chapter, the state of consciousness can also be inferred, though not directly measured, by analysis of electroencephalographic (EEG) information. Such analysis can be done by using either spectral-based techniques such as the "bispectral (BIS) index" or evoked potentials such as auditory evoked potentials. These indices do not directly measure unconsciousness or unresponsiveness. However, through extensive validation it is clear that these measures are predictive of the likelihood of response, provided that the anesthetic state has been induced with the drugs used to calibrate the electrophysiologic measure.
Nonresponsiveness can be introduced by deep sleep, an unusually boring chapter, or 2% isoflurane. What distinguishes the nonresponsiveness of the anesthetic state from the nonresponsiveness of normal sleep is the differential in stimulus that can penetrate the state of nonresponsiveness and rouse the brain to conscious perception. The hypnotic drugs used in anesthesia (propofol, thiopental, inhaled anesthetics, ketamine) are each capable of producing such profound CNS depression that even the most painful surgical stimulus cannot rouse patients from a state of nearly total nonresponsiveness. However, if the surgical stimulus can be attenuated before reaching the cortical level, less drug is required to maintain the state of nonresponsiveness.
Attenuation of surgical stimulation is the function of analgesic drugs (e.g., opioids) and local anesthetics. The interaction between analgesics and hypnotics is thus fundamental to understanding and defining anesthetic depth. Table 31-1 indicates the components of defining depth of anesthesia used in the remainder of this chapter.
Extending ideas proposed by Glass in 1998,[15]
consciousness can thus be perceived as a balance within the cortex between depression
and excitation ( Fig. 31-3, upper right
).
The cortex is primarily depressed by hypnotics, although opioids and nitrous oxide
also have sedating
| Afferent stimulus |
| Efferent response |
| Equilibrated concentrations of analgesic components |
| Equilibrated concentrations of hypnotic components |
| Equilibrated concentrations of other relevant drugs (e.g., β-blockers, muscle relaxants, local anesthetics) |
| Interaction surface relating drug concentrations to the probability of a given response to a given stimulus |
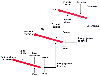 Figure 31-3
Stylized view of the interaction between hypnotics, which
depress consciousness, and analgesics, which suppress noxious stimuli.
Figure 31-3
Stylized view of the interaction between hypnotics, which
depress consciousness, and analgesics, which suppress noxious stimuli.
Figure 31-3 also shows the influence of opioids, nitrous oxide, and local anesthetics in preventing noxious stimulation from reaching the cortex. Systemic opioids act primarily on the midbrain and thalamus,[16] whereas neuraxial opioids act on the spinal cord. Local anesthetics act either at the spinal cord (for neuraxial blocks) or on peripheral nerves (e.g., nerve blocks and local infiltration). Nitrous oxide exerts some of its analgesic effects through spinal mechanisms,[17] [18] as well as midbrain mechanisms,[19] but for simplicity we will lump it together with opioids at the midbrain. The net effect of analgesic and local anesthetics is to attenuate the transmission of painful sensation to the cortex, thereby reducing the amount of hypnotic required to obtain a state of nonresponsiveness.
Figure 31-4 reduces the model in Figure 31-3 to a highly simplified pharmacologic view. The subcortical actions in Figure 31-3 have been compressed to a single site of action, the midbrain. Similarly, Figure 31-4 compresses all analgesics, including nitrous oxide, local anesthetics, and opioids, to just "opioids." Finally, Figure 31-4 equates "unconsciousness," which is clearly cortical, with "nonre-sponsiveness," which involves both cortical and subcortical structures. The result is a simplified pharmacologic structure that may provide insight into the interaction between analgesics and hypnotics on consciousness and responsiveness.
In Figure 31-4
,
painful stimuli arrive in the midbrain. Opioids attenuate the response. The magnitude
of the stimulus affects both the magnitude of the output and the apparent potency
of the opioids. Another way of saying this is that it takes more opioid to attenuate
an intensely painful stimulus than a modestly painful stimulus. This has been repeatedly
demonstrated, as shown in Figure 31-5
.
[20]
Pharmacologically, the effect of opioids on
attenuating the pain signal at the midbrain can be expressed by using a standard
sigmoid Emax pharmacodynamic equation:
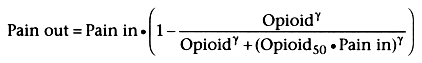
in which "pain in" is the afferent painful stimulus entering the midbrain, "pain
out" is the efferent painful stimulus transmitted to the cortex, "opioid" is the
opioid concentration in the midbrain, "opioid50
" is the equilibrated opioid
concentration associated with 50% attenuation of the "pain in" for an afferent pain
intensity of 1, and γ is the steepness of the opioid concentration-versus-response
relationship. Opioid50
is multiplied by "pain in" to reflect the decreased
potency of opioids in attenuating severe pain, as shown in Figure
31-5
.
In Figure 31-4
the output of the midbrain is projected to the cortex, where the CNS-arousing characteristics
of pain are balanced by the CNS-depressant effects of hypnotics. The pharmacologic
expression of this relationship would be
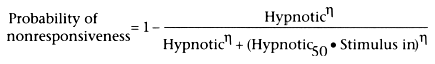
where "stimulus in" is the "pain out" projecting from the midbrain plus the ambient
stimulation, "hypnotic" is the concentration of the sedative-hypnotic in the cortex,
"hypnotic50
" is the concentration associated with
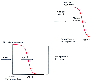 Figure 31-4
Pharmacologic interpretation of the interaction between
hypnotics and analgesics.
Figure 31-4
Pharmacologic interpretation of the interaction between
hypnotics and analgesics.
If we integrate these cascading models of drug effect, we get a response surface as shown in Figure 31-6 . The x and y axes of Figure 31-6 are the equilibrated concentrations of opioid and hypnotic, respectively, The z axis of Figure 31-6
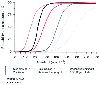 Figure 31-5
The effect of increasingly painful stimulation on the
opioid concentration-versus-response relationship.[20]
As pain intensity increases, the apparent potency of the opioids decreases.
Figure 31-5
The effect of increasingly painful stimulation on the
opioid concentration-versus-response relationship.[20]
As pain intensity increases, the apparent potency of the opioids decreases.
 Figure 31-6
Relationship between opioid and hypnotic drug concentrations
and the probability of nonresponsiveness. 1, No chance of nonresponsiveness; 2,
no chance of response; 3, hypnotic concentration-versus-response relationship in
the absence of opioids; 4, hypnotic concentration-versus-response relationship in
the presence of large doses of opioids; 5, area of maximum synergy between opioids
and hypnotics; 6, in the absence of hypnotics, even profound levels of opioids cannot
suppress the response; red line in the middle of
the surface, the 50% isobole; red curve at the top
of the surface, 95% isobole.
Figure 31-6
Relationship between opioid and hypnotic drug concentrations
and the probability of nonresponsiveness. 1, No chance of nonresponsiveness; 2,
no chance of response; 3, hypnotic concentration-versus-response relationship in
the absence of opioids; 4, hypnotic concentration-versus-response relationship in
the presence of large doses of opioids; 5, area of maximum synergy between opioids
and hypnotics; 6, in the absence of hypnotics, even profound levels of opioids cannot
suppress the response; red line in the middle of
the surface, the 50% isobole; red curve at the top
of the surface, 95% isobole.
Figure 31-6 is intentionally left unitless to illustrate that a very simple model of the anesthetic state, as shown in Figure 31-3 , generates a pharmacologic response surface that is very much like those seen in virtually all drug interaction trials.[28]
 Figure 31-7
Matrix of relevant stimuli and responses. Stimuli are
in approximately increasing order of noxiousness. Responses are in approximately
increasing order of difficulty of suppression. As the cells progress from left to
right and from top to bottom, increasingly larger doses of anesthetic are required
to suppress a given response to a given stimulus.
Figure 31-7
Matrix of relevant stimuli and responses. Stimuli are
in approximately increasing order of noxiousness. Responses are in approximately
increasing order of difficulty of suppression. As the cells progress from left to
right and from top to bottom, increasingly larger doses of anesthetic are required
to suppress a given response to a given stimulus.
The ability of inhaled anesthetics to induce immobility in response to noxious stimulation is mediated by the spinal cord, not the cortex.[29] [30] [31] We also know that intravenous opioids primarily work at the level of the midbrain. Experimentally, the interaction between inhaled anesthetics and opioids in preventing movement response to noxious stimulation looks very close to Figure 31-6 . Thus, it seems logical that downward projections from the midbrain to the spinal cord[32] are responsible for the MAC-sparing properties of opioids. As such, a pharmacologic model of the effect of opioids on MAC would resemble Figure 31-4 with a few changes to reflect opioids attenuating the signal at the dorsal horn and the response end point (movement) mediated by the spinal cord rather than the cortex.
To quantify anesthetic depth, we must be rigorous about the definition of the z axis in Figure 31-6 , nonresponsiveness. Consider a complex matrix of stimuli and responses, as shown in Figure 31-7 . Stimuli can be roughly divided into benign and noxious. Benign stimuli are not physically painful. Thus, responses to benign stimuli are readily suppressed by hypnotics alone, with minimal need for analgesic drugs. Noxious stimuli are physically painful, and thus responses to noxious stimuli are more readily suppressed in the presence of analgesics. Among the noxious stimuli, skin incision appears somewhat in the middle; it is more stimulating than electrical pain, but much less stimulating than laryngoscopy. Figure 31-7 presents the noxious stimuli in approximately increasing order.
Responses can be categorized as verbal, purposeful movement, involuntary movement, ventilation, hemo-dynamic response, sudomotor response, the formation of implicit and explicit memories, and EEG responses, as shown in Figure 31-7 . The responses also tend to follow a rank order in that loss of verbal response precedes loss of purposeful movement, which precedes loss of involuntary movement. Opioids tend to ablate hemodynamic response
For simplicity we will consider complete nonresponsiveness to be a lack of any of these responses. If we were interested only in consciousness, we might not include involuntary movement, ventilation response, hemodynamic response, or sudomotor response in our list of important responses because they can still occur in the absence of any conscious perception.
Figure 31-7 shows a matrix of stimuli and responses with 140 cells. Not all the cells are clinically relevant. For example, one rarely has involuntary movements, hyperventilates, or has autonomic responses to hearing one's name called, although beauty pageants and Internal Revenue Service visits are notable counterexamples. On the converse side, anesthesia should never be so inadequate that patients scream on incision. Thus, some cells in Figure 31-7 have been removed as being clinically uninteresting, which leaves 122 cells to define anesthetic depth. If we wanted to fully characterize the ability of isoflurane and fentanyl to create a state of nonresponsiveness for each of the listed responses to any of the listed stimuli, we would need to characterize the response surface ( Fig. 31-6 ) for each clinically relevant cell! With so many choices of stimuli and interactions, it is not surprising that anesthesiologists cannot agree on a simple definition of anesthetic depth. Fortunately, it is not really necessary to characterize the response to every stimulus. If we characterize the response to a benign stimulus, such as shaking and
 Figure 31-8
The role of opioid and hypnotics in the stimulus-response
relationship for three stimuli of increasing intensity and three increasingly difficult
responses to suppress. Each cell would have its own opioid-hypnotic interaction
surface, and three are displayed. The vertical axis is the probability of nonresponse.
Verbal nonresponsiveness to name calling is readily suppressed, even at light levels
of anesthesia. In contrast, profound levels of anesthesia are required to suppress
the hemodynamic response to intubation.
Figure 31-8
The role of opioid and hypnotics in the stimulus-response
relationship for three stimuli of increasing intensity and three increasingly difficult
responses to suppress. Each cell would have its own opioid-hypnotic interaction
surface, and three are displayed. The vertical axis is the probability of nonresponse.
Verbal nonresponsiveness to name calling is readily suppressed, even at light levels
of anesthesia. In contrast, profound levels of anesthesia are required to suppress
the hemodynamic response to intubation.
To this we must add the matrix of hypnotics versus opioids. Commonly used hypnotics include the anesthetic gases halothane, isoflurane, sevoflurane, and desflurane and the intravenous hypnotics propofol, thiopental, etomidate, and midazolam. Commonly used opioids include fentanyl, alfentanil, sufentanil, remifentanil, morphine, meperidine, hydromorphone, methadone, and (in Europe) tramadol and piritramide. With 8 hypnotics and 10 opioids, there are 80 combinations. Eighty combinations times 122 interactions yields 9760 combinations of opioids, hypnotics, stimuli, and responses. To this we must then add the influence of age, disease, genetics, and other factors. Clearly, it is impossible to create response surfaces to characterize how opioids and hypnotics produce a state of nonresponsiveness for all possible combinations of drugs, patients, stimuli, and responses.
Fortunately, there are powerful generalizations that provide both clinical insight and tractable experimental design. The most important is that for any stimulus-response pair, depth of anesthesia is the probability of nonresponse. More generally, depth of anesthesia is the probability of nonresponse to stimulation, calibrated against the strength of the stimulus, the difficulty of suppressing the response, and the drug-induced probability of nonresponsiveness. Anesthetic depth ranges from a 100% probability of an easily suppressed response (verbal answer) to a mild stimulus (e.g., calling one's name) and readily suppressed responses (e.g., verbal answer) to a 100% probability of nonresponse to profoundly noxious stimuli (e.g., intubation) and responses that are difficult to suppress (e.g., tachycardia). Table 31-1 lists the components needed to define anesthetic depth.
Figure 31-8 integrates the role of anesthetic drugs in the stimulus-response relationship. It shows three stimuli of
For the inhaled anesthetics, MAC creates a unifying principle. Although each inhaled anesthetic has some pharmacologic peculiarities, in general, they have parallel dose-response curves across drugs (e.g., isoflurane versus sevoflurane versus desflurane) and across stimuli-response pairs (MACawake ,[34] [35] MAC, MACBAR [36] [37] [MAC of anesthetic necessary to prevent the β-adrenergic response to skin incision]). Thus, by knowing the relative values of MAC, one can infer the relative values of the other stimulus-response relationships. There is less similarity among the intravenous hypnotics. Fortunately, only propofol and midazolam are commonly used to induce and maintain the anesthetic state, which limits the number of clinically interesting intravenous anesthetic combinations.
Despite conflicting reports of pharmacologic idiosyncrasies with each member of the fentanyl series, fentanyl, alfentanil, sufentanil, and remifentanil appear to differ mainly in potency, with otherwise parallel concentration-versus-response relationships. Morphine, meperidine, hydromorphone, and methadone appear to differ in both potency and intrinsic efficacy, with slightly lower maximal analgesic effect than noted with the fentanyl series of opioids. These latter four also have their own pharmacologic quirks, particularly meperidine.
Nitrous oxide is not only the oldest of the anesthetic drugs still in common use but remains one of the least well understood. It has properties of hypnotics, with an identifiable MAC of about 1 atm,[38] and is a modestly potent analgesic as well. Although the interaction of nitrous oxide with opioids,[39] inhaled anesthetics,[40] [41] and propofol[42] [43] has been characterized, no studies have gathered enough data to generate the response surfaces needed to fully understand the interactions of this nearly ubiquitous anesthetic drug.
Based on the simplifying assumptions, we need to understand a limited number of prototypic drug combinations: isoflurane-fentanyl, isoflurane-nitrous oxide, nitrous oxide-fentanyl, propofol-fentanyl, propofol-nitrous oxide, midazolam-nitrous oxide, and midazolam-fentanyl. For each of these combinations, characterization of the response surface for a handful of stimuli and responses would allow understanding of current clinical practice and provide for definition of the full range of anesthetic depth:
We now have a matrix of just 11 stimulus-versus-response relationships. Multiplication by our seven prototypic drug combinations yields 77 response surfaces, from which we can infer the rest of the surfaces through scaling based on MAC for inhaled drugs and C50 (plasma concentration that results in 50% drug effect) for intravenous drugs and the relative rankings of stimuli and responses. Moreover, the combinations range from light anesthesia, with modest stimulation and easily attenuated responses found in the upper right corner of Figure 31-7 , to deep anesthesia, with profound stimuli and very difficult to ablate responses found in the lower right corner of Figure 31-7 .
Figure 31-9 shows examples of response surfaces for a modest stimulus on the left (withdrawal in response to electrical tetanus) and a profound stimulus on the right (movement in response to intubation). These interaction surfaces are based on the same model as the interaction surface shown in Figure 31-6 ; they differ only in the intensity of the afferent painful signal. We find the same pattern when we examine real data. The left image in Figure 31-10 shows the response surface for MAC reduction of isoflurane by fentanyl. [23] The right image of Figure 31-10 shows the interaction of propofol with alfentanil on blunting all responses to intubation.[24] The shapes of these curves are not exactly like the shapes derived from our hypnotic-opioid interaction model because (1) the investigators were interested only in characterizing the interaction at the level of a 50% response and thus did not gather enough data to characterize the upper and lower edges of either curve and (2) the mathematical function used was multiple logistic regression, which has several
 Figure 31-9
Examples of response surfaces for a modest stimulus on
the left (withdrawal to electrical tetanus) and a
profound stimulus on the right (movement response
to intubation), based on the model shown in Figure
31-6
with differing intensity of the afferent painful signal. The figure
on the left shows a relatively mild stimulus-response
combination, whereas the figure on the right shows
a profoundly noxious stimulus-response combination.
Figure 31-9
Examples of response surfaces for a modest stimulus on
the left (withdrawal to electrical tetanus) and a
profound stimulus on the right (movement response
to intubation), based on the model shown in Figure
31-6
with differing intensity of the afferent painful signal. The figure
on the left shows a relatively mild stimulus-response
combination, whereas the figure on the right shows
a profoundly noxious stimulus-response combination.
Given our broad definition of anesthetic depth as providing nonresponsiveness to a wide range of stimuli and response pairs ( Figure 31-7 ), one might wonder what drugs constitute anesthetics. For example, one can envision an anesthetic consisting of β-blockers to blunt the hemodynamic response, trimethaphan to prevent tearing, a ventilator to control respiration, duct tape to prevent movement, and scopolamine to prevent memory. There is no chance of any response to any stimulation, so (1) is the patient deeply anesthetized? and (2) are β-blockers, trimethaphan, ventilators, muscle relaxants, scopolamine, and duct tape anesthetics?
The answer to both questions is "yes." For the first question, if the proposed technique could truly provide a 100% chance of nonresponse (and we haven't tried this personally), it would be clinically indistinguishable from
 Figure 31-10
The left image shows
the response surface for minimum alveolar concentration reduction of isoflurane by
fentanyl.[23]
The right image
shows the interaction of propofol with alfentanil on blunting all responses to intubation.
[24]
Both figures demonstrate the intrinsic synergy
between opioids and hypnotics in producing unresponsiveness.
Figure 31-10
The left image shows
the response surface for minimum alveolar concentration reduction of isoflurane by
fentanyl.[23]
The right image
shows the interaction of propofol with alfentanil on blunting all responses to intubation.
[24]
Both figures demonstrate the intrinsic synergy
between opioids and hypnotics in producing unresponsiveness.
|
|
|
|
|
|
|
|
|
|
|
|
|