|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Perioperative morbidity and mortality increase with increasing patient comorbidity. The original ASA physical status scoring system was proposed in 1941 and included six categories.[96] It was intended to standardize terminology and allow statistical analysis of outcomes between sites.[97] The original classification avoided the inclusion of surgical variables and was restricted to preoperative patient characteristics. It was revised in 1961 by Dripps and colleagues[46] to five categories, which were then adopted by the ASA.
The simplest example of such a relationship is the incidence of mortality correlated with ASA physical status. In their original study of perioperative mortality, Dripps and coworkers[46] demonstrated that mortality increased as severity of comorbid disease increased, as assessed by the ASA physical status classification. Several investigators have re-evaluated the relationship between operative mortality and ASA physical status classification. The studies by Pedersen[67] and Tiret[61] and their colleagues demonstrated such relationships. Vacanti and coworkers[98] also demonstrated the relationship between increasing mortality and decreasing physical status in 68,388 cases.
In Canada, Cohen and colleagues[51A] analyzed 100,000 anesthesia procedures and determined mortality within 7 days of operation using governmental vital statistics mortality data between the years 1975 and 1984. They established a computer database for each procedure that included age, preoperative conditions, ASA physical status, anesthesia technique, monitors, and other factors. The overall 7-day mortality rate was 71.04 deaths per 10,000 procedures. The mortality rate increased with advanced age, and it showed a marked increase among those older than 80 years. Rates were low for normal, healthy individuals and for those undergoing minor procedures. The investigators developed a multiple logistic regression model to determine the independent predictors of mortality. Significant risk markers for increasing mortality were advanced age, male gender, increasing physical status score, major or intermediate surgery, emergency procedure, having a complication in the operating room, narcotic anesthesia techniques, and having received only one or two anesthetic drugs ( Table 24-13 ).
In an attempt to look at each of the categories of contributing factors independently, Cohen's group[51A] constructed receiver-operator characteristic curves. There was no increment in prediction of mortality beyond that based on characteristics of the patient plus surgery. Plots including "other" or anesthesia-related factors showed almost complete overlap with curves including only patient- and surgery-specific factors.
One of the limitations of the ASA physical status classification is that ranking is a subjective measure conferred by the practitioner rather than an objective measure determined by the presence of specific disease states. Owens and coworkers[99] evaluated this hypothesis by asking 255 anesthesiologists to classify 10 hypothetical patients. In six of the cases, there was general agreement among the practitioners about classification of the patient; in the other four cases, there was divergence of opinion. The key finding of this study was that the ASA physical status classification is "useful but suffers from a lack of scientific precision."[99]
Rather than evaluating the risk of overall mortality, many studies have attempted to define the patient characteristics associated with morbidity and mortality related to a particular organ system. In evaluating the risk directly related to the patient's condition, it is important to understand the limitations of the methodology. All such studies evaluate the predictive value of a clinical or laboratory risk factor for a defined perioperative complication. In this approach, a cohort of individuals of interest is defined. In the optimal state, the study is performed prospectively, and the outcome of interest is assessed in a rigorous,
| Variable | All Procedures: Relative Odds of Dying within 7 Days | 95% Confidence Limits |
|---|---|---|
| Patient Related |
|
|
| Age (yr) |
|
|
| 60–79/<60 | 2.32 | 1.70, 3.17 |
| 80+/<60 | 3.29 | 2.18, 4.96 |
| Sex (F/M) | 0.77 | 0.59, 1.00 |
| Physical status score (3–5/1–2) | 10.65 | 7.59, 14.85 |
| Surgery Related |
|
|
| Major/minor | 3.82 | 2.50, 5.93 |
| Intermediate/minor | 1.76 | 1.24, 2.5 |
| Length of anesthesia (≤2 hr/<2 hr) | 1.08 | 0.77, 1.50 |
| Emergency/elective | 4.44 | 3.38, 5.83 |
| Other Factors |
|
|
| Year of operation (1975–1979/1980–1984) | 1.75 | 1.32, 2.31 |
| Had a complication in the operating or recovery room (yes/no) | 1.42 | 1.06, 1.89 |
| Anesthesia Related * |
|
|
| Experience of anesthetist (>600 procedures for ≥8 yr/<600 procedures for <8 yr) | 1.06 | 0.82, 1.37 |
| Inhalation with narcotic/inhalation alone | 0.76 | 0.51, 1.15 |
| Narcotic alone/inhalation alone | 1.41 | 1.01, 2.00 |
| Narcotic with inhalation/inhalation alone | 0.79 | 0.47, 1.32 |
| Spinal/inhalation alone | 0.53 | 0.29, 0.98 |
| No. of anesthetic drugs (1–2/3+) | 2.94 | 2.20, 3.84 |
| Adapted from Cohen MM, Duncan PG, Tate RB: Does anesthesia contribute to operative mortality? JAMA 260:2861, 1988. | ||
Many studies have adopted the approach of defining a cohort of individuals, determining their clinical and laboratory risk factors, and using multivariate modeling to determine the factors associated with increased risk. Frequently, perioperative risk is used to define intraoperative and postoperative complications. A major limitation in the use of multivariate modeling for this purpose is the assumption that the intraoperative period is a "black box" and that care is not modified by knowledge of the risk factor ( Fig. 24-5 ). However, anesthesiologists do modify their intraoperative care of high-risk patients in an attempt to reduce the risk. Changes in medical care over time and better knowledge about high-risk patients should result in reduction of risk related to specified clinical factors. For this reason, many of the indices developed previously may no longer have clinical validity.
In designing a multivariate risk index for perioperative morbidity and mortality, only factors that are included in the analysis and in the patient population of interest can be included in the final model. For example, if the
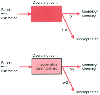 Figure 24-5
The concept of the black box
for risk indices. In developing a risk index, patients with a specific risk factor
enter the operating room and have a complication at a rate p.
If the anesthesiologist is aware of the importance of the risk factor and can modify
care to reduce such risk (p/2), the risk factor may
no longer be significant. If the risk factor is ignored, complications may again
occur in such patients.
Figure 24-5
The concept of the black box
for risk indices. In developing a risk index, patients with a specific risk factor
enter the operating room and have a complication at a rate p.
If the anesthesiologist is aware of the importance of the risk factor and can modify
care to reduce such risk (p/2), the risk factor may
no longer be significant. If the risk factor is ignored, complications may again
occur in such patients.
There are numerous specific disease states that increase perioperative risk. Cardiovascular disease is the most extensively studied, with the goal of identifying patients at greatest risk for fatal and nonfatal myocardial infarction. One of the earliest attempts to define cardiac risk was performed by Goldman and colleagues [13] at the Massachusetts General Hospital. They studied 1001 patients older than 45 years who were undergoing noncardiac surgery, excluding patients who underwent transurethral resection of the prostate under spinal anesthesia. Using multivariate logistic regression, they demonstrated nine clinical factors associated with increased morbidity and mortality. Each of these risk factors was associated with a given weight in the logistic regression equation, which was converted into points in the index. An increasing number of points was associated with increasing perioperative cardiac morbidity or mortality.
There have been several attempts to validate the Goldman Cardiac Risk Index. Zeldin[100] prospectively determined the Cardiac Risk Index for 1140 surgical patients and reported that the overall accuracy of the index was as high as in the original study, although the rate of complications in the highest-risk group was less than originally reported. Larsen and coworkers [101] also found that the Cardiac Risk Index demonstrated good accuracy in 2609 consecutive unselected patients older than 40 years. Domaingue and colleagues[102] studied patients undergoing various vascular surgery procedures and reported a higher probability of major cardiac complications than that reported by Goldman and coworkers[13] ; however, they also demonstrated a higher rate of complications with increasing cardiac risk class. The validity of the Cardiac Risk Index is more controversial for vascular surgery patients. Jeffrey and colleagues[103] evaluated the rate of cardiac complications in 99 patients undergoing elective abdominal aortic surgery and demonstrated a similar pattern of increased overall complication rates with increasing cardiac risk. A higher percentage (7%) of patients in the lowest category in their study sustained a cardiac complication. White and colleagues[104] demonstrated the value of the Goldman Cardiac Risk Index for long-term survival after vascular surgery. Several other studies were unable to demonstrate any relationship between the Cardiac Risk Index and perioperative cardiac complications, with a high incidence of complications found in patients with a Cardiac Risk Index of I or II. [105] [106] When the ASA physical status classification was compared with the Goldman Cardiac Risk Index in a cohort of 16,277 patients undergoing noncardiac surgery,[107] both indices demonstrated predictive value, although the objective Goldman Cardiac Risk Index had little increased value over the more subjective ASA physical status classification.
Other investigators have attempted to develop risk indices. Detsky and coworkers[108] studied a cohort of individuals who were referred to an internal medicine service for preoperative evaluation. Many of the factors identified by Golman[13] were confirmed or slightly modified in the Detsky index, and angina was added to the risk factors. The researchers advocated the calculation of a pretest probability of complication based on the type of surgery, after which the Detsky Modified Risk Index is applied with the use of a nomogram. In this manner, the overall probability of complications can be determined as a function of the surgical procedure and of patient disease. The Detsky index was advocated as the starting point for risk stratification in the American College of Physicians Guideline on preoperative evaluation.[109] In an attempt to update the original index, Lee and colleagues[110] at the Brigham and Women's Hospital studied 4315 patients 50 years or older who were undergoing elective major noncardiac procedures in a tertiary-care teaching hospital. Six independent predictors of complications were identified and included in a Revised Cardiac Risk Index: high-risk type of surgery, history of ischemic heart disease, history of congestive heart failure, history of cerebrovascular disease, preoperative treatment with insulin, and preoperative serum creatinine level higher than 2.0 mg/dL. The rate of major cardiac complications increased with the number of risk factors.
Numerous risk indices developed for cardiac surgery have been developed.[111] [112] [113] [114] [115] [116] The goal has been to identify patients who are at greatest risk for adverse perioperative outcomes, not specifically anesthesia-related risk. Many of these indices focus on anatomic considerations for perioperative risk. They are extremely useful in risk adjustment to assess rates of mortality. For example, the State of New York annually publishes data on mortality rates associated with coronary bypass grafting by surgeon and by hospital.[117] [118] [119] For comparison of rates across institutions, risk figures must be adjusted so that institutions that perform predominantly high-risk surgery are not penalized.
Patients undergoing vascular surgery represent a cohort with very high perioperative complication rates.[120] Clinical risk indices and noninvasive diagnostic testing have been used to identify patients with the highest rates of perioperative mortality and major morbidity. Numerous studies demonstrated that the presence of a reversible defect on thallium imaging and new regional wall motion abnormalities on dobutamine echocardiography predicts those with a high perioperative risk.[121] [122] [123] [124] [125] [126] [127] [128] [129] [130] As use of these technologies spread, the tests lost much of their predictive value because they were used in lower-risk patients.[131] [132] Subsequent studies demonstrated that the tests should be applied only to patients who are at moderate or high risk and identified a group of clinical characteristics that define the group for whom the test can have optimal predictive characteristics. Eagle and colleagues[133] determined the value of clinical risk factors for predicting perioperative cardiac events and the additive value of noninvasive testing based on the preoperative risk profile. Five clinical predictors were identified: age older than 70 years, diabetes mellitus, angina, ventricular ectopic activity being treated, and Q waves on an electrocardiogram. Among patients
Vanzetto and coworkers[135] performed a prospective evaluation in which an expanded number of clinical variables was identified in a cohort of patients undergoing major vascular surgery. Cardiovascular morbidity and mortality was determined prospectively in this population. An increasing number of clinical variables was associated with increasing perioperative risk. Most importantly, dipyridamole thallium imaging was performed only in the subset of patients with two or more clinical variables, and the results of the test were not made available to the clinicians caring for the patients. In this well-performed study, the presence of thallium redistribution identified a cohort with an incrementally greater perioperative risk compared with those with negative scan results and a similar number of clinical variables.
Similar to the work with dipyridamole thallium imaging, dobutamine stress echocardiography can be further quantified. The presence of wall motion abnormalities at a slow heart rate is the best predictor of increased perioperative risk; large areas of defect are of secondary importance.[130] Boersma and colleagues[136] assessed the value of dobutamine stress echocardiography with respect to the extent of wall motion abnormalities and use of β-adrenergic blockers during surgery (see Chapter 27 ). They assigned one point for each of the following characteristics: age older than 70 years, current angina, myocardial infarction, congestive heart failure, prior cerebrovascular accident, diabetes mellitus, and renal failure. As the total of number of clinical risk factors increases, perioperative cardiac event rates also increase. Dobutamine stress echocardiography was performed only for patients with a significant number of risk factors, and patients who demonstrated new wall motion abnormalities had higher event rates than those with the same clinical risk score but without new wall motion abnormalities.
As part of their study of anesthesia-related mortality, Pedersen and colleagues[67] evaluated the occurrence of cardiovascular and pulmonary complications. For a total of 7306 anesthesia procedures, they reported a 6.3% incidence of intraoperative or postoperative cardiovascular complications and a 4.8% incidence of intraoperative or postoperative pulmonary complications. Acute myocardial infarction occurred in 0.16% of the patients. The investigators evaluated a number of characteristics, including gender, age, presence of ischemic heart disease, duration of anesthesia, and type of surgery, and assessed their relationship to cardiovascular and pulmonary complications and overall mortality in the hospital ( Table 24-14 ). Cardiopulmonary complications were associated with advanced age (>70 years), preoperative signs of ischemic heart disease with recent myocardial infarction, chronic heart failure, chronic lung disease, and abdominal surgery, particularly of an emergency nature. They observed that the use of pancuronium was associated with worse perioperative outcome, a finding reminiscent of the curare-related deaths of the 1940s and 1950s.
Numerous studies have evaluated the importance of single variables such as hypertension on perioperative risk. Goldman and Caldera[137] evaluated a cohort of patients undergoing noncardiac surgery with general anesthesia. Hypertension did not denote a group with increased perioperative risk, although the number of patients with diastolic blood pressure greater than 110 mm Hg was insufficient to draw any conclusions. This study highlighted the problems related to generalization of results, because many subsequent researchers have suggested that surgery be delayed for patients with a diastolic blood pressure greater than 110 mm Hg. In one of the few studies to demonstrate a relationship, Hollenberg and coworkers[138] identified hypertension and the presence of left ventricular hypertrophy as predictors of perioperative ischemia, but they did not consider their independent relationship with perioperative major morbidity.
Examples of a prospective cohort study to identify the risk of a particular clinical factor include studies of the rate of perioperative reinfarction in patients who sustained a previous myocardial infarction. Traditionally, risk assessment for noncardiac surgery was based on the time interval between the myocardial infarction and surgery. Multiple studies have demonstrated an increased incidence of reinfarction if the myocardial infarction occurred within 6 months of surgery. [14] [15] [16] With improvements in perioperative care, this difference has decreased.
The previous example of assessing risk illustrates the issue of changes in management over time. The importance of the intervening time interval may no longer be valid in the current era of thrombolytics, angioplasty, and risk stratification after acute myocardial infarction. Although many patients with myocardial infarction continue to have myocardium at risk for subsequent ischemia and infarction, in others, the critical area of coronary stenosis is totally occluded or widely patent. For example, the use of percutaneous transluminal coronary angioplasty (PTCA) is associated with a reduced incidence of death or reinfarction within 6 months.[139] Patients should be evaluated from the perspective of their risk for ongoing ischemia. In their guidelines for perioperative evaluation of noncardiac surgery, the American Heart Association/American College of Cardiology Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures[140] proposed that patients who have had a myocardial infarction within less than 30 days should be considered the group at highest risk; after that period, risk stratification is based on disease severity and exercise tolerance.
|
|
|
|
|
|
|
|
|
|
|
|
|