ISSUES RELATED TO STUDY DESIGN
Types of Studies
To interpret the literature, it is important to understand the
strengths and limitations of the various study designs. Prospective
cohort studies involve the identification of a group of subjects who are
monitored over time for the occurrence of an outcome of interest. The goal is to
identify patients who develop the outcome. For studies of perioperative mortality,
individual cases can be reviewed to determine the cause of mortality.
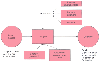 Figure 24-1
Representation of the influences of various components
on poor perioperative outcomes. Surgical, anesthetic, and patient characteristics
all contribute to outcome. Anesthesia-related contributions can include issues of
judgment and mishaps, as well as characteristics of the provider. The surgical procedure
itself affects outcome, as does the location of intraoperative and postoperative
care.
Figure 24-1
Representation of the influences of various components
on poor perioperative outcomes. Surgical, anesthetic, and patient characteristics
all contribute to outcome. Anesthesia-related contributions can include issues of
judgment and mishaps, as well as characteristics of the provider. The surgical procedure
itself affects outcome, as does the location of intraoperative and postoperative
care.
Alternatively, data on all patients in the cohort can be obtained, and factors that
are associated with the development of morbidity or mortality can be discerned.
An example of a prospective cohort study identifying factors associated with perioperative
cardiac morbidity and mortality is that of Goldman and colleagues,[13]
which led to the development of the Cardiac Risk Index.
Another example of a prospective cohort study is one in which
patients with a known disease are studied for the development of predefined outcomes.
Such studies provide the natural history of the disease. For example, studies of
patients who have sustained a myocardial infarction could be important in determining
the optimal time between the infarct and surgery.[14]
[15]
[16]
Although prospective cohort studies have important value in identifying
risk factors for the outcome of interest, there are significant limitations. The
selection of the cohort of interest can significantly affect the results obtained.
The larger the cohort, the more the results can be generalized. A second bias is
that many patients may be lost to follow-up. In perioperative studies, this may
not be an important issue for short-term outcomes. The importance of a risk factor
depends on the completeness of the data. For example, if the presence of severe
angina were not included in the database, it could not be considered as a risk factor,
and other factors may appear to be more important.[13]
A specific example of a prospective cohort study is the randomized
clinical trial. Randomized clinical trials represent the gold standard
for evidence of causation. They have defined inclusion and exclusion criteria, treatment
protocols, and outcomes of interest. They are usually single- or double-blinded
(to patient and physician) studies and are designed to test the effect of a new drug
or intervention. They rarely are used as a means of identifying risk, but they may
be used to determine whether a risk factor is truly linked by a causal relationship
or is simply an association. For example, hypothermia in the perioperative period
has been associated with an increased incidence of perioperative ischemia, a surrogate
marker for morbidity.[17]
In a randomized clinical
trial, the use of forced-air warming to maintain normothermia was associated with
a significantly lower incidence of perioperative morbid cardiac events.[18]
Randomized clinical trials directed at interventions that reduce the frequency of
a given risk factor in the population are frequently performed after the results
of a prospective cohort study to confirm the findings of an association between poor
outcome and that factor.
Randomized clinical trials derive their strength from an evidence-based
perspective because of their high degree of internal validity; the randomization
scheme and use of placebo (or accepted alternative treatments) provide strong evidence
that the results are related to the intervention. Importantly, these trials have
a lower degree of external validity because the intervention may not behave in the
same manner when it is diffused in a more heterogeneous population in whom treatment
is not defined.
Retrospective studies involve
identification of patients who have sustained an outcome and definition of risk factors
associated with the outcome. An example of a retrospective design is a case-control
study. Case-control studies identify patients with the outcome of interest.
Frequently, these patients are included as part of a prospective cohort study.
The prevalence of a risk factor in the patients with the outcome (i.e., cases) is
compared with the prevalence of the risk factor in matched controls to maximize the
efficiency and power of the results. The ratio of cases to controls can be varied,
yielding greater power with an increasing number of controls.
Case-control studies are subject to several biases. The exact
definitions of cases and controls can influence the analysis. Frequently, patients
are matched for age and gender, but other factors may play an important role. It
is critical that the exposure precedes the outcome, and a dose-response gradient
further confirms the relationship.
 Figure 24-1
Representation of the influences of various components
on poor perioperative outcomes. Surgical, anesthetic, and patient characteristics
all contribute to outcome. Anesthesia-related contributions can include issues of
judgment and mishaps, as well as characteristics of the provider. The surgical procedure
itself affects outcome, as does the location of intraoperative and postoperative
care.
Figure 24-1
Representation of the influences of various components
on poor perioperative outcomes. Surgical, anesthetic, and patient characteristics
all contribute to outcome. Anesthesia-related contributions can include issues of
judgment and mishaps, as well as characteristics of the provider. The surgical procedure
itself affects outcome, as does the location of intraoperative and postoperative
care.