|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Renal hemodynamic function is defined by RBF, RPF, intrarenal distribution of flow, FF, and renal vascular resistance. Glomerular function is defined by the GFR. Tubular functions include concentrating ability, water conservation, and sodium conservation. For practical purposes, however, clinical tests of renal function can be described as those based on clearance techniques (the most commonly used) and those that test tubular function. A number of tests are also primarily used for clinical and laboratory investigation.
Renal function is indirectly assessed by clearance techniques,
[11]
which are based on the Fick principle. The
amount of substance x excreted by the kidney equals
the amount delivered in the arterial supply minus the amount in venous return:
Excretionx
=
Deliveryx
− Returnx
(2)
 Figure 20-8
Tubular concentration of urine. The juxtamedullary nephrons
have long loops of Henle associated with the vasa recta. Dashed
arrows represent passive movement of fluid or solutes along concentration
or osmolar gradients; solid arrows represent active
transport. Tubular fluid enters the distal proximal tubule iso-osmotic with plasma
(300 mOsm/kg) (1). In the descending limb of Henle (2), water rapidly diffuses out
into the increasingly hypertonic medulla and is removed by the vasa recta, and as
a consequence, the tubular fluid becomes hypertonic, largely because of concentration
of sodium chloride (NaCl). Urea diffuses in from the hypertonic interstitium, further
increasing tubular fluid osmolality (1200 mOsm/kg). In the thin ascending loop of
Henle (3), NaCl passively diffuses into the interstitium along its concentration
gradient, but water is trapped in the water-impermeable tubule, which progressively
decreases tubular fluid osmolality. Urea passively diffuses into the tubular fluid
(urea recycling). Tubular dilution is accelerated by active reabsorption of NaCl
in the thick ascending loop (the diluting segment) and proximal distal tubule (4).
The fluid entering the distal tubule is quite hypo-osmotic (100 mOsm/kg). In the
collecting segment (5), the osmolality of the tubular fluid returns to that of plasma
(300 mOsm/kg), but unlike the contents of the proximal tubule, the solute component
consists largely of urea, creatinine, and other excreted compounds. Increased plasma
antidiuretic hormone (ADH) renders the cortical and medullary collecting ducts (6)
permeable to water, which passively diffuses into the hypertonic medullary interstitium.
Even though some urea diffuses out into the medulla, the maximal osmolality of concentrated
urine (7) approaches that of the hypertonic medullary interstitium, about 1200 mOsm/kg.
In the absence of ADH, the collecting ducts remain impermeable to water, and the
urine is diluted. (From Stanton BA, Koeppen BM: Control of body fluid osmolality
and volume. In Berne RM, Levy MN [eds]: Physiology,
4th ed. St Louis, CV Mosby, 1998, pp 715–743.)
Figure 20-8
Tubular concentration of urine. The juxtamedullary nephrons
have long loops of Henle associated with the vasa recta. Dashed
arrows represent passive movement of fluid or solutes along concentration
or osmolar gradients; solid arrows represent active
transport. Tubular fluid enters the distal proximal tubule iso-osmotic with plasma
(300 mOsm/kg) (1). In the descending limb of Henle (2), water rapidly diffuses out
into the increasingly hypertonic medulla and is removed by the vasa recta, and as
a consequence, the tubular fluid becomes hypertonic, largely because of concentration
of sodium chloride (NaCl). Urea diffuses in from the hypertonic interstitium, further
increasing tubular fluid osmolality (1200 mOsm/kg). In the thin ascending loop of
Henle (3), NaCl passively diffuses into the interstitium along its concentration
gradient, but water is trapped in the water-impermeable tubule, which progressively
decreases tubular fluid osmolality. Urea passively diffuses into the tubular fluid
(urea recycling). Tubular dilution is accelerated by active reabsorption of NaCl
in the thick ascending loop (the diluting segment) and proximal distal tubule (4).
The fluid entering the distal tubule is quite hypo-osmotic (100 mOsm/kg). In the
collecting segment (5), the osmolality of the tubular fluid returns to that of plasma
(300 mOsm/kg), but unlike the contents of the proximal tubule, the solute component
consists largely of urea, creatinine, and other excreted compounds. Increased plasma
antidiuretic hormone (ADH) renders the cortical and medullary collecting ducts (6)
permeable to water, which passively diffuses into the hypertonic medullary interstitium.
Even though some urea diffuses out into the medulla, the maximal osmolality of concentrated
urine (7) approaches that of the hypertonic medullary interstitium, about 1200 mOsm/kg.
In the absence of ADH, the collecting ducts remain impermeable to water, and the
urine is diluted. (From Stanton BA, Koeppen BM: Control of body fluid osmolality
and volume. In Berne RM, Levy MN [eds]: Physiology,
4th ed. St Louis, CV Mosby, 1998, pp 715–743.)
The amount of substance x delivered to the kidney is the product of the arterial plasma concentration (Pax ) and RBF. The amount returning from the kidney is the product of the venous plasma concentration (Pvx ) and RBF. The urinary excretion rate of substance x is the product of its urinary concentration (Ux ) and the urine flow rate in milliliters per minute (V).
Therefore,
(Pax
× RBF)
= (Pvx
× RBF) + (Ux
× V) (4)
However, in practice, RBF and venous return are not measured.
Instead, the removal of substance x from plasma
by the kidney is expressed in the concept of clearance. Clearance (C) is defined
as the virtual volume of plasma cleared of substance x
per unit time, in milliliters per minute. This term allows the urinary excretion
rate of x to be equated to its renal arterial plasma
concentration:
Pax
× C =
Ux
× V (5)
If the assumption is made that the concentration of x
in renal arterial and venous plasma is identical, clearance of substance x
may be calculated by using a urine sample, arm venous sample, and measured urinary
flow rate:
Cx
= (Ux
× V)Pax
(6)
PAH is an organic anion that is almost completely cleared from plasma in a single pass through the kidney by a combination of glomerular filtration and proximal
Effective renal blood flow (eRBF) may be derived if the hematocrit
level (Hct) is known and expressed as a decimal (i.e., 35% = 0.35):
eRBF = eRPF/(1 − Hct) (8)
In a number of circumstances CPAH provides misleading information about RPF. If the plasma PAH concentration exceeds the tubular maximum for reabsorption of 12 mg/dL, the excess PAH is returned to the renal vein, the secreted fraction declines, and RPF is underestimated.[2] About 80% of PAH is cleared by tubular secretion, so if proximal tubule function deteriorates, PAH clearance declines and again underestimates RPF.
These errors can be overcome if arterial (Pa) and renal vein (Pv)
PAH levels are accessible. The renal extraction of PAH (EPAH
) can be
calculated and provides an assessment of proximal tubule function:
EPAH
= (PaPAH
− PvPAH
)/PaPAH
(9)
When renal function is normal, renal vein PAH is close to zero
and PAH extraction approaches 1.0. As proximal tubule function declines, the concentration
of PAH in the renal vein increases, and PAH extraction progressively decreases below
1.0. True RPF is calculated by dividing PAH clearance by PAH extraction:
RPF = CPAH
/EPAH
(10)
In the presence of hypovolemia and oliguria, PAH is sequestered in the kidney. Even with the use of extraction techniques, PAH clearance may misrepresent RPF under these conditions. Despite its relative convenience as an experimental tool, PAH clearance may be an unreliable indicator of RBF during the perturbations induced by anesthesia and surgical stress.
Inulin is an inert polyfructose sugar that is completely filtered
by the glomerulus and is neither secreted nor reabsorbed by the renal tubules. The
volume in milliliters of plasma cleared of inulin per minute represents the GFR (mL/min).
Inulin clearance is measured identically to PAH clearance. After an intravenous
loading dose of 30 to 50 mg/kg, a continuous infusion of inulin is given to establish
a steady-state plasma concentration of 15 to 20 mg/dL. The bladder is usually flushed
with air to eliminate any pooled urine. A very carefully timed urine collection
is then made, which can be as short as 30 minutes. It is generally accepted that
inulin clearance (CIn
) provides the most accurate available determination
of GFR:
GFR = CIn
= (UIn
×
V)/PIn
(11)
Although inulin clearance is the "gold standard" for measurement of GFR, it is seldom used clinically because its accurate measurement is laborious and requires meticulous attention to detail. The inulin assay is time consuming, and inulin itself is in short supply because of lack of demand. Inulin meets all the criteria of an ideal filtration marker, but large changes in blood glucose during the test may interfere with its measurement, and its accuracy in reflecting the GFR cannot be directly assessed, only inferred. The predicted variability of inulin clearance is 20% when measurements are compared at two different times in the same individual and 40% when measurements are compared between two individuals.[11] Normal values for inulin clearance are 110 to 140 mL/min/1.73 m2 (males) and 95 to 125 mL/min/1.37 m2 (females).
FF is the fraction of RPF that is filtered by the glomerulus:
FF = GFR/RPF = CIn
/CPAH
(12)
Normally, the GFR is about 125 mL/min and RPF is about 660 mL/min, so FF approximates 125/660, or about 0.2. Changes in FF are thought to represent changes in periglomerular arteriolar tone (see the earlier section "Afferent and Efferent Arteriolar Control Mechanisms"). An increase in FF indicates that the GFR is increased relative to RPF. This increase could be achieved by efferent arteriolar constriction or afferent arteriolar dilation and maintains glomerular filtration pressure in the face of decreased RPF. Conversely, a decrease in FF implies that the GFR is decreased relative to RPF by afferent arteriolar constriction or efferent arteriolar dilation.
Creatinine, the endogenous end product of creatine phosphate metabolism,
is normally generated from muscle at a very uniform rate and is handled by the kidney
in a manner similar to that of inulin. Thus, creatinine clearance (CCr
)
provides a simple, inexpensive bedside estimate of GFR. A single blood sample is
drawn at the midpoint of a carefully timed urine collection:
GFR = CCr
= (UCr
×
V)/PCr
(13)
Bedside use of creatinine clearance has been restricted by the belief that a prolonged
(12- to 24-hour) urine collection is necessary to eliminate error induced by residual
urine in the bladder neck after spontaneous voiding, a practice both tedious and
cumbersome. The estimated creatinine clearance depends on when the blood sample
is drawn, so if serum creatinine changes rapidly during
The precise timing, not the duration, of the urine collection is the critical issue.[12] If a brisk urine flow is induced by diuresis and care is taken to empty the bladder, the variability in creatinine clearance is no greater with a 1-hour urine collection than with a 24-hour collection. In catheterized patients with urine flow rates greater than 15 mL/hr, creatinine clearance obtained with a 2-hour urine collection gives values equivalent to those obtained with a 12- to 24-hour collection.[13] Moreover, a short urine collection enables rapid, repeated estimates of GFR to be obtained. This not only makes 2-hour creatinine clearance a viable bedside test in critically ill patients but also implies that a changing GFR can be closely tracked by serial estimations of creatinine clearance ( Fig. 20-9 , Fig. 20-10 , and Fig. 20-11 ). For example, in trauma patients, a 1-hour creatinine clearance of less than 25 mL/min determined within 6 hours of surgery reliably predicted postoperative acute renal failure despite the absence of oliguria.[14]
Considerable variation in the normal range of creatinine clearance has been noted. Tobias and coauthors[12] reported a variation in creatinine clearance between 88 and 148 mL/min and in serum creatinine between 0.9 and 1.5 mg/dL in a single healthy individual over a period of 5 years. There is also a diurnal variation, with higher values in the afternoon and a variance of up to 25% around mean values.[15] It is prudent to obtain short-collection creatinine clearance estimates at the same time each day to minimize diurnal variability. "Normal" creatinine clearance is related to body surface area and weight, so values may fluctuate widely in patients with cachexia or edema.
 Figure 20-9
Creatinine clearance: 2- versus 22-hour values. There
is a close and significant correlation in creatinine clearance estimates from a 2-hour
and 22-hour urine collection. CC02, 2-hour urine collection; CC22, 22-hour urine
collection. (From Sladen RN, Endo E, Harrison T: Two-hour versus 22-hour
creatinine clearance in critically ill patients. Anesthesiology 67:1013–1016,
1987.)
Figure 20-9
Creatinine clearance: 2- versus 22-hour values. There
is a close and significant correlation in creatinine clearance estimates from a 2-hour
and 22-hour urine collection. CC02, 2-hour urine collection; CC22, 22-hour urine
collection. (From Sladen RN, Endo E, Harrison T: Two-hour versus 22-hour
creatinine clearance in critically ill patients. Anesthesiology 67:1013–1016,
1987.)
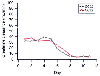 Figure 20-10
New-onset acute renal failure. In a patient in whom
acute renal failure is developing in the intensive care unit, the exponential decline
in creatinine clearance is tracked equally well whether a 2-hour (CC02) or a 22-hour
(CC22) urine collection is used. However, data from the 2-hour collection are available
well before those from the 22-hour collection.
Figure 20-10
New-onset acute renal failure. In a patient in whom
acute renal failure is developing in the intensive care unit, the exponential decline
in creatinine clearance is tracked equally well whether a 2-hour (CC02) or a 22-hour
(CC22) urine collection is used. However, data from the 2-hour collection are available
well before those from the 22-hour collection.
Creatinine clearance has a number of inherent limitations even if collection error is carefully avoided. The creatinine generation rate varies with muscle mass, physical activity, protein intake, and catabolism. The most commonly used serum creatinine assay is the Jaffé reaction, which is based on the red color of the creatinine complex with alkaline picrate. It also measures other normally occurring chromogens, such as glucose, protein, ketones, and ascorbic acid, which represent about 14% of total creatinine when renal function is normal, though substantially less when serum creatinine is elevated. Ketoacidosis, barbiturates, and cephalosporin antibiotics may artifactually increase serum creatinine by as much as 100% and falsely decrease measured creatinine clearance.
Unlike inulin, about 20% of creatinine is secreted by the proximal tubule, so creatinine clearance overestimates
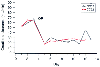 Figure 20-11
Renal revascularization in a patient with renovascular
hypertension and renal insufficiency admitted to the intensive care unit for preoperative
monitoring and stabilization. Bilateral renal revascularization was performed, and
after return from the operating room, a substantial decline in renal function was
noted. These changes were tracked equally well by creatinine clearance derived from
a 2-hour (CC02) and a 22-hour (CC22) collection.
Figure 20-11
Renal revascularization in a patient with renovascular
hypertension and renal insufficiency admitted to the intensive care unit for preoperative
monitoring and stabilization. Bilateral renal revascularization was performed, and
after return from the operating room, a substantial decline in renal function was
noted. These changes were tracked equally well by creatinine clearance derived from
a 2-hour (CC02) and a 22-hour (CC22) collection.
For all these reasons, an isolated creatinine clearance estimate may not be diagnostic of lesser degrees of renal dysfunction. Nonetheless, serial estimates of creatinine clearance provide a useful clinical guide to alterations in renal function and prognosis. The variability of creatinine clearance diminishes as the GFR declines; in fact, loss of variability is a clue to deteriorating renal function. If the GFR is rapidly declining, creatinine clearance alerts the physician earlier and more compellingly than serum creatinine does because it reflects the creatinine excretion rate (i.e., urine creatinine content times urine flow rate [UCr × V]). Directional changes between creatinine clearance and inulin clearance show good agreement.[16] At low GFR levels, a creatinine-inulin clearance ratio as high as 2:1 (e.g., 12 versus 6 mL/min) would induce little actual difference in clinical management.
Serum creatinine is a useful marker of stable renal function, but it is unreliable when the GFR is rapidly changing.[17] The serum creatinine concentration depends on its volume of distribution (total-body water), creatinine generation rate (muscle mass and rate of catabolism), and creatinine excretion rate (GFR). Perioperative fluid administration increases total body water and dilutes serum creatinine, which underestimates renal dysfunction. In a cachectic patient with very low muscle mass, creatinine generation may be so feeble that the serum creatinine level remains subnormal even in the face of a markedly decreased GFR. In some patients with a serum creatinine concentration of less than 0.9 mg/dL, creatinine clearance may be less than 25 mL/min.[18]
The relationship between serum creatinine and GFR is inverse and exponential. A doubling of serum creatinine implies a halving of the GFR. An increase in serum creatinine from 0.8 to 1.6 mg/dL may not generate much attention, but it indicates a 50% decrease in the GFR. A much larger increase from 4 to 8 mg/dL also represents a 50% decrease in GFR, but by this time renal insufficiency is well established ( Fig. 20-12 ). After a transient renal insult (e.g., suprarenal aortic cross-clamping), serum creatinine may increase for a few days while the GFR is actually recovering.[19] In oliguric acute renal failure, serum creatinine is directly proportional to the creatinine generation rate. Creatinine clearance is reliably low but is associated with wide variability in serum creatinine.
 Figure 20-12
Relationship between serum creatinine and glomerular
filtration rate (GFR). The relationship between serum creatinine and GFR as measured
by creatinine clearance is reciprocal and exponential. Doubling of serum creatinine
corresponds to halving of the GFR. Relatively large declines in GFR from normal
are associated with small increases in serum creatinine until the GFR decreases below
60 mL/min; further decrements are associated with large increases in serum creatinine.
(From Alfrey AC, Chan L: Chronic renal failure: Manifestations and pathogenesis.
In Schrier RW [ed]: Renal and Electrolyte Disorders,
4th ed. Boston, Little, Brown, 1992, p 541.)
Figure 20-12
Relationship between serum creatinine and glomerular
filtration rate (GFR). The relationship between serum creatinine and GFR as measured
by creatinine clearance is reciprocal and exponential. Doubling of serum creatinine
corresponds to halving of the GFR. Relatively large declines in GFR from normal
are associated with small increases in serum creatinine until the GFR decreases below
60 mL/min; further decrements are associated with large increases in serum creatinine.
(From Alfrey AC, Chan L: Chronic renal failure: Manifestations and pathogenesis.
In Schrier RW [ed]: Renal and Electrolyte Disorders,
4th ed. Boston, Little, Brown, 1992, p 541.)
Cockroft and Gault[20] formulated a nomogram for rapid estimation of creatinine clearance without urine collection. The nomogram was based on population studies incorporating serum creatinine, age, weight, and gender.
For males,
CCr
= (140 − Age) × Weight in kg/(Serum
creatinine × 72) (14)
For females, the derived creatinine clearance is multiplied by 0.85.
In these formulas, the body weight that is used may substantially alter the derived creatinine clearance. In obese or edematous patients, total body weight is much greater than the lean body mass from which creatinine is derived, and creatinine clearance is overestimated. In cachectic patients with depleted lean body mass, creatinine production is so low that serum creatinine is frequently less than 1.0 mg/dL and overestimates the true GFR. Robert and associates[21] adjusted the Cockroft-Gault equation to incorporate ideal body weight from a nomogram and serum creatinine corrected to 1.0 mg/dL (if less than
When renal function is rapidly changing, serum creatinine-based nomograms are subject to the same limitations as serum creatinine itself. Rapid alterations in GFR are reflected by rapid changes in the creatinine excretion rate, which is incorporated into measured creatinine clearance as UCR × V. Serum creatinine itself changes much more slowly and depends on the equilibrium point between creatinine production and excretion. In fact, serum creatinine does not begin to increase above normal levels until the GFR declines below 50 mL/min/1.73 m2 , and occasionally it will remain normal even when the GFR dips as low as 20 to 40 mL/min/1.73 m2 .
|
|
|
|
|
|
|
|
|
|
|
|
|