|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Until recently, nervous control of the vasculature was considered predominantly in terms of classic transmitters: norepinephrine and acetylcholine. For many years, they were the only transmitters recognized in perivascular nerves.
 Figure 16-2
The diagram shows that adenosine triphosphate (ATP) and
norepinephrine (NE) are released as cotransmitters from the sympathetic nerves supplying
the vas deferens and some blood vessels. ATP acts on P2
-purinoceptors
on the smooth muscles to initiate excitatory junction potentials, action potentials,
and a fast initial contraction involving electromechanical coupling through voltage-dependent
calcium (Ca2+
) channels. NE acts on α1
-adrenoceptors
to produce the second, slower phase of the contraction by pharmacomechanical (or
at least spike-independent) coupling through receptor-operated Ca2+
channels.
Prejunctional α2
-adrenoceptors and P1
-purinoceptors
can reduce transmitter release when activated by NE and adenosine (AD), respectively
(i.e., prejunctional neuromodulation), whereas NE and ATP enhance each other's actions
(i.e., postjunctional neuromodulation). (Adapted from Burnstock G: Local
mechanisms of blood flow control by perivascular nerves and endothelium. J Hypertens
Suppl 8:S95, 1990.)
Figure 16-2
The diagram shows that adenosine triphosphate (ATP) and
norepinephrine (NE) are released as cotransmitters from the sympathetic nerves supplying
the vas deferens and some blood vessels. ATP acts on P2
-purinoceptors
on the smooth muscles to initiate excitatory junction potentials, action potentials,
and a fast initial contraction involving electromechanical coupling through voltage-dependent
calcium (Ca2+
) channels. NE acts on α1
-adrenoceptors
to produce the second, slower phase of the contraction by pharmacomechanical (or
at least spike-independent) coupling through receptor-operated Ca2+
channels.
Prejunctional α2
-adrenoceptors and P1
-purinoceptors
can reduce transmitter release when activated by NE and adenosine (AD), respectively
(i.e., prejunctional neuromodulation), whereas NE and ATP enhance each other's actions
(i.e., postjunctional neuromodulation). (Adapted from Burnstock G: Local
mechanisms of blood flow control by perivascular nerves and endothelium. J Hypertens
Suppl 8:S95, 1990.)
The concepts of cotransmission and neuromodulation have become accepted mechanisms in autonomic nervous control. To establish that transmitters coexisting in the same nerves act as cotransmitters, it is necessary to demonstrate that, on release, each substance acts postjunctionally on its own specific receptor to produce a response.
For many perivascular sympathetic nerves, there is evidence that norepinephrine and ATP act as cotransmitters and are released from the same nerves but act on α1 -adrenoceptors and P2 -purinoceptors, respectively, to produce vasoconstriction[6] [7] ( Fig. 16-2 ). ATP, once thought to act only as an electrical buffer for the charged norepinephrine, is believed to mediate contraction through P2x -receptors by voltage-dependent calcium channels.[8] The fast component of contraction appears to be mediated by these purinoceptors, whereas norepinephrine sustains contraction of muscle by acting on the α1 -adrenoreceptor through receptor-operated calcium channels. Specific drugs have been designed to interact with this purinergic component.[9]
Neuromodulators modify the process of neurotransmission. They may be circulating neurohormones, local agents, or neurotransmitter substances released from the same nerves or from others nearby. Neuromodulation can occur prejunctionally by decreasing or increasing the amount of transmitter released during transmission or
NPY is also colocalized with norepinephrine and ATP. However, in some vessels, NPY has little or no direct action; instead, NPY acts as a neuromodulator prejunctionally to inhibit the release of norepinephrine from the nerve or postjunctionally to enhance the action of norepinephrine ( Fig. 16-3A ).[13] [14] In other vessels, notably those of the spleen, skeletal muscle, and cerebral and
 Figure 16-3
Schematic representation of different interactions that
occur between neuropeptide Y (NPY) and adenosine triphosphate (ATP), and norepinephrine
(NE) released from single sympathetic nerve varicosities. A,
Diagram shows what occurs in the vas deferens and many blood vessels, where NE and
ATP, probably released from small granular vesicles, act synergistically to contract
(+) the smooth muscle through α1
-adrenoceptors and P2
-purinoceptors,
respectively. B and C,
Sympathetic neurotransmission in the heart and brain (B)
and spleen (C). (From Lincoln J, Burnstock
G: Neural-endothelial interactions in control of local blood flow. In
Warren J [ed]: The Endothelium: An Introduction to Current Research. New York,
Wiley-Liss, 1990, p 21.)
Figure 16-3
Schematic representation of different interactions that
occur between neuropeptide Y (NPY) and adenosine triphosphate (ATP), and norepinephrine
(NE) released from single sympathetic nerve varicosities. A,
Diagram shows what occurs in the vas deferens and many blood vessels, where NE and
ATP, probably released from small granular vesicles, act synergistically to contract
(+) the smooth muscle through α1
-adrenoceptors and P2
-purinoceptors,
respectively. B and C,
Sympathetic neurotransmission in the heart and brain (B)
and spleen (C). (From Lincoln J, Burnstock
G: Neural-endothelial interactions in control of local blood flow. In
Warren J [ed]: The Endothelium: An Introduction to Current Research. New York,
Wiley-Liss, 1990, p 21.)
A classic transmitter such as acetylcholine coexists with VIP in the parasympathetic nerves of many organs, but in this instance, the two transmitters are stored in separate vesicles. They can be released differentially at different stimulation frequencies, depending on where they are located.[16] [17] For example, in the salivary gland, they can act independently on acinar cells and glandular blood vessels ( Fig. 16-4 ).[7] Cooperation is achieved by the selective release of acetylcholine at low frequencies and of VIP at high frequencies of stimulation. Elements of prejunctional and postjunctional modulation have also been described. It is becoming increasingly apparent that in many biologic states, including pregnancy,[18]
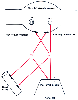 Figure 16-4
A classic transmitter, acetylcholine (ACh), coexists
with vasoactive intestinal polypeptide (VIP) in parasympathetic nerves supplying
the cat salivary gland. ACh and VIP are stored in separate vesicles; they can be
released differentially at different stimulation frequencies to act on acinar cells
and glandular blood vessels. Cooperation is achieved by the selected release of
ACh at low impulse frequencies and of VIP at high frequencies. Prejunctional and
postjunctional modulation is indicated. (From Burnstock G: Local mechanisms
of blood flow control by perivascular nerves and endothelium. J Hypertens Suppl
8:S95, 1990.)
Figure 16-4
A classic transmitter, acetylcholine (ACh), coexists
with vasoactive intestinal polypeptide (VIP) in parasympathetic nerves supplying
the cat salivary gland. ACh and VIP are stored in separate vesicles; they can be
released differentially at different stimulation frequencies to act on acinar cells
and glandular blood vessels. Cooperation is achieved by the selected release of
ACh at low impulse frequencies and of VIP at high frequencies. Prejunctional and
postjunctional modulation is indicated. (From Burnstock G: Local mechanisms
of blood flow control by perivascular nerves and endothelium. J Hypertens Suppl
8:S95, 1990.)
|
|
|
|
|
|
|
|
|
|
|
|
|