|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Transdermal drug delivery generally requires high solubility in both water and oil, low molecular weight, high potency, and little or no skin irritation. Fentanyl is available in a transdermal delivery system. Potential advantages of delivering fentanyl transdermally include no first-pass drug metabolism by the liver; improved patient compliance, convenience, and comfort; and consistent analgesia. Doses of transdermal fentanyl include 20, 50, 75, and 100 µg/hour and achieve blood levels ranging from less than 1.0 to 2.0 ng/mL, although significant variability exists.[417] [418] Plasma fentanyl levels rise and usually reach a plateau within 8 to 16 hours. The half-life for the decline in fentanyl levels after patch removal is high (17 ± 2.3 hour) ( Fig. 11-30 ).[419] Elevated body temperature accelerates either the release of fentanyl from the patch or the distribution from the subcutaneous fat depot.
Results of clinical trials utilizing transdermal fentanyl for postoperative analgesia have demonstrated a high incidence of significant respiratory depression, and this application is not recommended.[417] [418] [420] Transdermal fentanyl is effective in cancer pain management. Patients should have analgesia provided by faster-acting opioid preparations and then be converted to transdermal fentanyl, with allowances made for drug conversions.
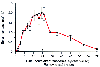 Figure 11-30
Serum fentanyl concentration change after placement of
the transdermal fentanyl system. (From Varvel JR, Shafer SL, Hwang SS, et
al: Absorption characteristics of transdermally administered fentanyl. Anesthesiology
70:928–934, 1989.)
Figure 11-30
Serum fentanyl concentration change after placement of
the transdermal fentanyl system. (From Varvel JR, Shafer SL, Hwang SS, et
al: Absorption characteristics of transdermally administered fentanyl. Anesthesiology
70:928–934, 1989.)
Iontophoresis is a technique by which drug passage through the skin is augmented by an external electric current. Clinically significant doses of morphine and fentanyl can be delivered iontophoretically.[421]
Similar to transdermal drug delivery, transmucosal delivery through the oropharynx and nasopharynx eliminates hepatic first-pass metabolism (drugs are absorbed directly into the systemic circulation) and improves patient comfort, convenience, and compliance.
Buprenorphine, a potent, synthetic morphine analog with mixed opioid agonist-antagonist properties and a long half-life, is readily absorbed from sublingual mucosal tissues. The portion of the drug that is swallowed is almost completely metabolized by the liver, and only a small fraction can reach the systemic circulation when swallowed. Systemic bioavailability after sublingual buprenorphine is approximately 50% of that following intravenous administration. In several studies, sublingual buprenorphine (0.4 mg) was compared with conventional intramuscular morphine or meperidine and found to provide comparable and satisfactory analgesia.[422]
Initial experience with buccal morphine for postoperative analgesia had been promising. Bell and colleagues demonstrated that buccal morphine has a 50% bioavailability.[423] However, subsequent studies by Fisher and associates questioned the reliability and systemic availability of buccal morphine.[424] The low lipid solubility of morphine makes it an unlikely candidate for effective transmucosal absorption. Opioids with high lipid solubility, such as buprenorphine, fentanyl and methadone are more effectively absorbed sublingually than those with low lipid solubility, such as morphine ( Fig. 11-31 ). [425]
Oral transmucosal fentanyl citrate (OTFC) is a solid dosage form of fentanyl that consists of fentanyl incorporated into a sweetened lozenge on a stick. A portion of fentanyl is absorbed through the oral mucosa, and the rest is swallowed and absorbed through the gastrointestinal tract. Swallowed fentanyl has a low bioavailability
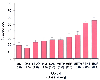 Figure 11-31
The mean absorption of opioids after 10 minutes in the
oral cavity of normal subjects. The pH of the dosing solution was 6.5. MS, morphine
sulfate; OXY, oxycodone; LEVO, levorphanol; HM, hydromorphone; NAL, naloxone; METH,
methadone; HER, heroin; FENT, fentanyl; BUP, buprenorphine. (From Weinberg
DS, Inturrisi CE, Reidenberg B, et al: Sublingual absorption of selected opioid
analgesics. Clin Pharmacol Ther 44:335–342, 1988.)
Figure 11-31
The mean absorption of opioids after 10 minutes in the
oral cavity of normal subjects. The pH of the dosing solution was 6.5. MS, morphine
sulfate; OXY, oxycodone; LEVO, levorphanol; HM, hydromorphone; NAL, naloxone; METH,
methadone; HER, heroin; FENT, fentanyl; BUP, buprenorphine. (From Weinberg
DS, Inturrisi CE, Reidenberg B, et al: Sublingual absorption of selected opioid
analgesics. Clin Pharmacol Ther 44:335–342, 1988.)
Delivery of opioids through the nasal mucosa has also been investigated. Although intranasal sufentanil (1.5, 3.0, 4.5 µg/kg) in children allows easy separation from parents, many of the children cry on drug administration.[434] Side effects of intranasal sufentanil in children include reduced ventilatory compliance (chest wall rigidity), hypoxemia, impaired positive pressure ventilation by mask, and nausea and vomiting.[435] [436] In adults, too, 10 to 15 µg of intranasal sufentanil induces moderate sedation with few side effects 20 to 40 minutes after administration.[437] Bioavailability of intranasal sufentanil is 78% of intravenous sufentanil. Butorphanol, a partial
Morphine (10 mg) and fentanyl (300 µg) inhalation produces low plasma drug levels (∼10 and 0.2–0.4 ng/mL, respectively) and analgesia that may be disproportionately greater than expected.[441] [442] Inhaled liposome-encapsulated fentanyl was also demonstrated to be a noninvasive route of administration with a rapid increase and prolonged maintenance of plasma fentanyl concentration.[443]
The rectal mucosa is another site for transmucosal drug delivery. Morphine is only poorly absorbed from the rectal mucosa of children. However, sustained-release morphine hydrogel suppositories (MHS) may be more promising.[444] Plasma morphine levels after MHS were lower than after intramuscular morphine, but linear analog scales for pain were lower, and the incidence of nausea was reduced after rectal sustained-release therapy.[444] The hydrogel formulation of rectal morphine may be useful for premedication and analgesia in pediatric patients.[445]
Despite the high first-pass metabolism of opioid analgesics, morphine has been formulated into an oral, sustained-release tablet (MST) and has been evaluated for premedication,[446] postoperative analgesia, [447] [448] and anaglesia for chronic cancer pain.[449] MSTs provide unreliable preoperative anxiolysis and postoperative pain relief, possibly because of delayed time to onset of peak effects (3 to 5 hours), which can be increased by impaired gastric emptying and absorption from the small intestine. As an analgesic for chronic cancer pain, MSTs were shown to be an excellent formula.[449] A controlled-release formulation of oxycodone administered every 12 hours was more effective for postoperative analgesia than immediate-release oxycodone in patients undergoing anterior cruciate ligament repair.[450]
|
|
|
|
|
|
|
|
|
|
|
|
|