Nontherapeutic Effects
Endogenous opioid peptides are widely distributed in brainstem
nuclei regulating respiration. All μ receptor-stimulating opioids cause dose-dependent
depression of respiration in humans primarily through a direct action on brainstem
respiratory centers.[159]
How the various respiratory
centers involved with ventilatory drive, respiratory rhythm generation, chemoreception,
and neural integration are affected by opioids is unclear.
The stimulatory effect of CO2
on ventilation is significantly
reduced by opioids. The hypercapneic response can be separated into the central
component and the peripheral component. Morphine-induced changes in the central
component were equal in men and women, whereas changes in the peripheral component
were greater in women.[160]
In addition, the apneic
threshold and resting end-tidal PCO2
are
increased by opioids ( Fig. 11-8
).
Opioids also decrease hypoxic ventilatory drive.
The respiratory rate is usually drastically slowed in opioid overdose,
although hypoxic CNS insult can counter this effect. The prolonged expiratory time
in the respiratory cycle induced by opioids frequently results in greater reductions
in respiratory rate than in tidal volume. A recent report demonstrated that monitoring
of breath intervals can sensitively detect fentanyl-induced respiratory depression
and can be used as a measure of dynamic opioid effect.[161]
High doses of opioids usually eliminate spontaneous respirations without necessarily
producing unconsciousness. Patients receiving high doses of opioids may still be
responsive to verbal commands and often will breathe when directed to do so.
The peak onset of respiratory depression after an analgesic dose
of morphine is slower than after comparable doses of fentanyl: 30 ±
15 minutes versus 5 to 10 minutes. Respiratory depression induced by
small doses of morphine usually lasts longer than after equipotent doses of fentanyl.
Sufentanil (0.1–0.4 µg/kg) produces shorter-lasting respiratory depression
and longer-lasting analgesia than fentanyl (1.0–4.0 µg/kg).[162]
Plasma fentanyl concentrations of 1.5 to 3.0 ng/mL are usually associated with significant
decreases in CO2
responsiveness. With higher doses of fentanyl (50–100
µg/kg), respiratory depression can persist for many hours. When moderately
large (20–50 µg/kg) or greater doses of fentanyl are used, the potential
need for postoperative mechanical ventilation should be anticipated. Although adequate
spontaneous
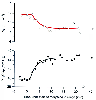 Figure 11-8
Influence of morphine administration (bolus dose of 100
µg/kg given at time 0 minutes, followed by a continuous infusion of 30 µg/kg/hour)
on resting inspired minute ventilation and resting pressure of end-tidal CO2
(PET
CO2
) in a single subject. A one-component exponential
was fitted to the data. The estimated time constant for the Vi
data is
3.0 minutes and for PET
CO2
data is 2.6 minutes. The time delays
are between 1 and 2 minutes. (From Sarton E, Teppema L, Dahan A: Sex differences
in morphine-induced ventilatory depression reside within the peripheral chemoreflex
loop. Anesthesiology 90:1329–1338, 1999.)
Figure 11-8
Influence of morphine administration (bolus dose of 100
µg/kg given at time 0 minutes, followed by a continuous infusion of 30 µg/kg/hour)
on resting inspired minute ventilation and resting pressure of end-tidal CO2
(PET
CO2
) in a single subject. A one-component exponential
was fitted to the data. The estimated time constant for the Vi
data is
3.0 minutes and for PET
CO2
data is 2.6 minutes. The time delays
are between 1 and 2 minutes. (From Sarton E, Teppema L, Dahan A: Sex differences
in morphine-induced ventilatory depression reside within the peripheral chemoreflex
loop. Anesthesiology 90:1329–1338, 1999.)
ventilation after alfentanil-N2
O is likely with plasma alfentanil concentrations
of less than 200 ng/mL,[163]
significant residual
respiratory depression can exist at lower levels. The effects of remifentanil, no
matter what the dose, are attenuated rapidly and completely within 5 to 15 minutes
following termination of its administration. In healthy humans, the EC50
for depression of minute ventilation with remifentanil and alfentanil was 1.17 ng/mL
and 49.4 ng/mL, respectively[164]
Although the mechanism by which pain modulates ventilatory control
is unknown, clinical daily practice provides indirect evidence that pain stimulates
ventilation, especially during emergence from anesthesia. Combes and coworkers demonstrated
that pain relief with nerve block in patients after knee surgery, who were previously
treated with morphine PCA, increases the incidence of abnormal respiratory events
associated with oxygen desaturation.[165]
Naloxone
has been accepted as a standard therapy for opioid-induced respiratory depression.
However, there have been reports of naloxone-resistant respiratory depression after
intrathecal or epidural opioids.[166]
[167]
 Figure 11-8
Influence of morphine administration (bolus dose of 100
µg/kg given at time 0 minutes, followed by a continuous infusion of 30 µg/kg/hour)
on resting inspired minute ventilation and resting pressure of end-tidal CO2
(PET
CO2
) in a single subject. A one-component exponential
was fitted to the data. The estimated time constant for the Vi
data is
3.0 minutes and for PET
CO2
data is 2.6 minutes. The time delays
are between 1 and 2 minutes. (From Sarton E, Teppema L, Dahan A: Sex differences
in morphine-induced ventilatory depression reside within the peripheral chemoreflex
loop. Anesthesiology 90:1329–1338, 1999.)
Figure 11-8
Influence of morphine administration (bolus dose of 100
µg/kg given at time 0 minutes, followed by a continuous infusion of 30 µg/kg/hour)
on resting inspired minute ventilation and resting pressure of end-tidal CO2
(PET
CO2
) in a single subject. A one-component exponential
was fitted to the data. The estimated time constant for the Vi
data is
3.0 minutes and for PET
CO2
data is 2.6 minutes. The time delays
are between 1 and 2 minutes. (From Sarton E, Teppema L, Dahan A: Sex differences
in morphine-induced ventilatory depression reside within the peripheral chemoreflex
loop. Anesthesiology 90:1329–1338, 1999.)