DROPERIDOL
History
General anesthesia with inhaled anesthetics and barbiturates depresses
the entire CNS in a nonspecific manner. Laborit and Huguenard[706]
[707]
in the 1950s sought an anesthetic technique
that would produce "artificial hibernation" devoid of circulatory and respiratory
depression. Their concept was to use drugs that would produce neurovegetative blockade
(multifocal inhibition) of the cellular, autonomic, and endocrine mechanisms normally
activated in response to stress.[708]
The first
attempt at developing this concept was the lytic cocktail, which contained an analgesic
(meperidine), two tranquilizers (chlorpromazine and promethazine), and atropine.
Although this combination of drugs did enjoy widespread use for conscious sedation,
it produced respiratory depression and was not used for general anesthesia. Janssen
[709]
synthesized haloperidol, the first member
of
the butyrophenones, and it became the primary neuroleptic component in neuroleptanesthesia
(NLAN). DeCastro and Mundeleer[710]
in 1959 combined
haloperidol with phenoperidine (a meperidine derivative also synthesized by Janssen)
in a forerunner to the practice of NLAN. Droperidol, a derivative of haloperidol,
and fentanyl, a phenoperidine congener, both synthesized by Janssen, were used by
DeCastro and Mundeleer[710]
in a combination that
they reported to be superior to haloperidol and phenoperidine. This NLAN combination
produced more rapid onset of analgesia, less respiratory depression, and fewer extrapyramidal
side effects. The fixed combination of droperidol and fentanyl, marketed as Innovar
in the United States, was the drug primarily used for NLAN. The use of NLAN has
largely disappeared in modern anesthetic practice. The primary use of droperidol
in anesthesia has been as an antiemetic and to a lesser extent as a sedative and
antipruritic. The present package insert for droperidol in the United States carries
a black box warning regarding the potential for fatal arrhythmias and recommendations
that it be administered only with continuous electrocardiographic monitoring. However,
with the withdrawal of droperidol in certain countries and more stringent labeling
regarding potentially lethal dysrhythmias in others, the use of droperidol has decreased
tremendously. The validity of the risk of low-dose droperidol causing QT prolongation,
dysrhythmias, and death has been challenged by numerous editorials, articles, and
letters reviewing the cases that prompted this action.[711]
[712]
[713]
[714]
[715]
[716]
Droperidol is a butyrophenone, a fluorinated derivative of the
phenothiazines ( Fig. 10-27
).
[708]
Butyrophenones produce CNS depression characterized
by marked apparent tranquility and cataleptic immobility. They are potent antiemetics.
Droperidol is a potent butyrophenone, and like the others, it produces its action
centrally at sites where dopamine, norepinephrine, and serotonin act. It has been
postulated that butyrophenones may occupy GABA receptors on the postsynaptic membrane,
thereby reducing synaptic transmission and resulting in a build-up of dopamine in
the intersynaptic cleft.[708]
[709]
In particular, droperidol results in submaximal inhibition of the α1
-,
β1
-, and γ2
-subunits of GABAA
and full
inhibition of α2
- acetylcholine receptors. This submaximal inhibition
of GABA receptors by droperidol may explain the anxiety, dysphoria, and restlessness
that can occur with its administration.[717]
An
imbalance in dopamine and acetylcholine is thought to occur and result in an alteration
in normal transmission of signals in the CNS. The chemoreceptor trigger zone is
the emetic center, and "red" astrocytes transport neurolept molecules from the capillary
to dopaminergic synapses in the chemoreceptor trigger zone, where they occupy GABA
receptors ( Fig. 10-28
).
This is thought to be
 Figure 10-27
Structure of droperidol, a butyrophenone derivative.
Figure 10-27
Structure of droperidol, a butyrophenone derivative.
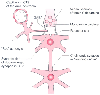 Figure 10-28
Site of action of droperidol. The mode of action of
neuroleptanesthetics is at the chemoreceptor trigger zone (CTZ). GABA, γ-aminobutyric
acid. (Redrawn with modification from Janssen PAJ: Pharmacological aspects.
In Bente D, Bradley P [eds]: Neuropsychopharmacology.
Amsterdam, Elsevier, 1965, p 151.)
Figure 10-28
Site of action of droperidol. The mode of action of
neuroleptanesthetics is at the chemoreceptor trigger zone (CTZ). GABA, γ-aminobutyric
acid. (Redrawn with modification from Janssen PAJ: Pharmacological aspects.
In Bente D, Bradley P [eds]: Neuropsychopharmacology.
Amsterdam, Elsevier, 1965, p 151.)
the mechanism by which droperidol exerts its antiemetic effect.[718]
 Figure 10-27
Structure of droperidol, a butyrophenone derivative.
Figure 10-27
Structure of droperidol, a butyrophenone derivative. Figure 10-28
Site of action of droperidol. The mode of action of
neuroleptanesthetics is at the chemoreceptor trigger zone (CTZ). GABA, γ-aminobutyric
acid. (Redrawn with modification from Janssen PAJ: Pharmacological aspects.
In Bente D, Bradley P [eds]: Neuropsychopharmacology.
Amsterdam, Elsevier, 1965, p 151.)
Figure 10-28
Site of action of droperidol. The mode of action of
neuroleptanesthetics is at the chemoreceptor trigger zone (CTZ). GABA, γ-aminobutyric
acid. (Redrawn with modification from Janssen PAJ: Pharmacological aspects.
In Bente D, Bradley P [eds]: Neuropsychopharmacology.
Amsterdam, Elsevier, 1965, p 151.)