|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Respiratory failure may be defined as inadequate oxygenation resulting in systemic arterial hypoxemia or inadequate ventilation resulting in systemic arterial hypercapnia (see Chapter 59 ). Because it is impossible to ascribe specific ABG values to this definition without knowledge of the clinical situation, respiratory failure is best described by an integration of historical, clinical, and laboratory observations. These observations must be tailored to the pediatric patient.
A complete family history and medical history characterizing the severity and chronicity of the respiratory dysfunction help in formulating a differential diagnosis and a therapeutic approach. Specific data should include a history of prematurity, previous airway instrumentation, previous mechanical ventilation, nonpulmonary organ dysfunction, and a family history of any respiratory disease. A detailed feeding history and up-to-date growth chart can provide valuable information.
Clinical examination of lung function includes close visual observation as well as auscultation. The color of the skin and mucous membranes should be documented, as well as the frequency, pattern, and depth of respiration. The presence of any end-expiratory grunting, nasal flaring, and intercostal or subcostal retractions can be used to estimate the work of breathing. The profound compliance of the infant's chest wall can exaggerate the severity of retractions. The symmetry of chest movement should be noted visually; because of the small thoracic volume with easy transmission of sounds, auscultation is not always the best way to ascertain symmetry of air entry. Abdominal distention should also be assessed. Because children are abdominal breathers and have very compliant chest walls, abdominal distention can dramatically impede adequate air entry. The sounds produced by air movement through the airways can be examined by naked ear as well as by the stethoscope.
Radiologic evaluation of the nasopharynx, neck, and thorax can often provide meaningful information regarding the cause and severity of the respiratory dysfunction. Fluoroscopy can be used as an adjunct in evaluating an uncooperative child.
Pulmonary function tests are frequently useful for assessing respiratory function, but because of lack of cooperation, these tests are difficult to perform in any child younger than 5 years. Numerous maneuvers may be used to evaluate an unintubated child; most of them require a tight-fitting mask, which in itself can be problematic.[115] Lung volumes, expiratory flow rates, compliance, and inspiratory forces can be measured in an intubated child; in fact, most ventilators routinely provide these measurements.
Measurement of ABGs (see Chapter 41 ) is an objective indicator of the adequacy of gas exchange. PaO2 is helpful, but it is most useful when considered in relation to the inspired oxygen content. Two ways of evaluating this relationship are the gradient difference between the partial pressure of inspired oxygen and PaO2 (A-a gradient) and calculation of shunting of desaturated blood across the lung.
Elimination of CO2 from arterial blood is another indicator of lung function. The specific importance of PaCO2 must always be considered in light of the finding that CO2 is more diffusible than oxygen and any increase in minute ventilation can often compensate for inefficiency of CO2 excretion.
Various invasive as well as noninvasive techniques permit measurement of alveolar PO2 (PAO2 ), PaO2 , PACO2 , and PaCO2 in even the smallest infants and children. ABG sampling can be thought of as the gold standard. When numerous or repeat analyses are required, blood sampling can be facilitated by an indwelling arterial cannula.
Umbilical artery cannulation is still a popular technique in the newborn; these catheters are relatively simple to insert and, once in place, are easy to maintain. They are most commonly used in a very-low-birth-weight infant of less than 1000 g or in a larger infant during initial resuscitation before transport to an intensive care nursery. To reduce the risk of thromboembolic complications to the renal and splanchnic circulations, the tip of the catheter should be in the aorta either at the level of the diaphragm or at the level of the aortic bifurcation. Extreme caution must be used because all intra-arterial catheters carry the risk of distal thromboembolic disease, and a catheter in the aorta has the potential for distal infarction of extremities or vital organs.[116] Insertion of an arterial catheter as distal from the central circulation as possible would seem to offer the potential advantage of minimal embolic damage. In fact, peripheral arterial cannulation has become the most common technique for repeat ABG sampling in infants and children.[117]
With proper insertion and maintenance, serious complications of arterial lines can be avoided. Although many arteries cannulated on a long-term basis are occluded after removal of the cannula, these arteries tend to recanalize within a short period.[118]
Less invasive techniques for monitoring gas exchange have also been developed. Transcutaneous electrodes designed to measure oxygen and CO2 are inaccurate during hypoperfusion states, and transcutaneous monitors require a warm-up time that makes "spot checks" difficult. They are better used as trend monitors after correlating the value with a measured arterial sample. Absolute values can be misleading, particularly with the CO2 electrode.[119] [120] The use of pulse oximeters is routine in the care of critically ill children. They are accurate, do not require any warm-up time, and need no skill for application. Unlike fingertip or toe placement on a larger patient, the probe is usually wrapped around the child's entire hand or foot.[121] End-tidal CO2 monitoring allows continuous assessment of CO2 elimination, and the capnogram (CO2 excretion recorded through time) provides information about gas flow in the small and large airways. This technology has limitations in a small child: dead space is increased, the weight of the monitor on the end of the ETT can result in accidental extubation, and the volume of gas withdrawn for sampling can alter the inspired volume and inspired oxygen fraction (FIO2 ) in an extremely small child.[122]
The cause of respiratory failure can be differentiated by the age of the patient at initial evaluation. Respiratory failure in a newborn often reflects a congenital abnormality or immaturity. Congenital abnormalities can include airway malformations, lung dysgenesis, or nonpulmonary organ dysgenesis or malfunction, all of which impair ventilation. Lesions of immaturity include both apnea of prematurity and hyaline membrane disease, a biochemical immaturity of the surfactant system. In addition, the perinatal period is characterized by specific infections, insults, and stress reactions. One particular problematic reaction is persistent pulmonary hypertension, which can complicate neonatal pulmonary and nonpulmonary problems. These and other important causes of respiratory failure in the newborn are listed in Table 76-8 . A wide variety of disorders can cause respiratory failure in older children ( Table 76-9 ). Regardless of the specific cause, respiratory failure can be categorized as hypoventilation syndromes in patients with normal lungs, intrinsic alveolar and interstitial disease, and obstructive airway disease.
Clinical conditions that produce hypoventilation include neuromuscular disease, central hypoventilation on the basis of reduced CNS efferent activity, and structural/anatomic impairment of lung expansion (i.e., upper airway obstruction, massive abdominal distention). These clinical conditions are characterized by inadequate lung expansion, which secondarily results in progressive atelectasis (intrapulmonary right-to-left shunt) with systemic hypoxia. Atelectasis and the resultant decreased FRC also increase the work of breathing. The child's response to this increased work of breathing at lower lung volumes is to breathe faster with reduced tidal volume at lower lung volumes, a pattern that causes further atelectasis and shunting. As a result, children with intrinsically normal lungs but with hypoventilation syndromes present with shallow tachypnea and possible evidence of increased work of breathing and cyanosis. Their radiographs reveal small lung volumes with miliary or lobar atelectasis. Lung re-expansion with intermittent positive-pressure ventilation and PEEP quickly reverses the pathologic process.
Intrinsic lung disease involving the alveoli or pulmonary interstitium affects respiratory function by decreasing lung compliance and increasing airway closure, both resulting in increased work of breathing and atelectasis. Edema or inflammation of the alveoli and edema, inflammation, or fibrosis of the interstitium decrease the compliance of the lung or make the lung stiffer. In a stiffer lung, a greater negative intrapleural pressure is required to generate air movement, thus increasing the work of breathing.
In addition, the normal developmental physiology of large closing volumes with a tendency to airway closure is exaggerated and results in airway closure, increasing atelectasis, and shunting. The principal pathophysiologic feature in children with intrinsic alveolar or pulmonary interstitial disease is atelectasis. Such a child may clinically and radiographically resemble a child with normal lungs and a hypoventilation syndrome, but with a different response to therapeutic maneuvers.
Obstruction of the airways can be extrinsic, such as lower tracheal and upper bronchial compression by vascular structures or neoplasms, or intrinsic, caused by intraluminal obstruction or by the airway wall itself. Intrinsic small airway obstruction is commonly seen in bronchiolitis, bronchopneumonia, asthma, and bronchopulmonary dysplasia.
The decreased conductance or increased resistance increases the work of breathing. The degree of airway obstruction ranges from partial to complete. Partial obstruction impedes air outflow more than air inflow and therefore results in trapped gases or regional emphysema. Complete airway obstruction results in atelectasis. In disease of the small airways, there is usually a mixture of total and partial obstruction throughout the airways that results in an inhomogeneous picture of collapse and overdistention. The areas of collapse contribute to an intrapulmonary right-to-left shunt, and the areas of overdistention contribute to dead space. If overdistention is the predominant feature, overdistention of the entire lung can occur and result in decreased compliance and increased work of breathing. The key to small airways disease is the inhomogeneity of the pathophysiology; the
|
|
Congenital Abnormalities | Developmental Immaturity | Specific Neonatal "Stress" |
|---|---|---|---|
| Impaired control of ventilation | Central nervous system dysgenesis | Apnea of prematurity | Drug intoxication (note maternal drugs) |
|
|
Ondine's curse | Intracranial hemorrhage | Sepsis |
|
|
|
|
Central nervous system infections |
|
|
|
|
Seizures |
| Neuromuscular disorders | Congenital myopathies |
|
High cervical cord injuries |
| Structural impairment | Thoracic deformities |
|
Severe abdominal distention |
|
|
Lung hypoplasia |
|
Pneumothorax or other air leak |
|
|
Diaphragmatic hernia |
|
|
|
|
Potter's syndrome |
|
|
|
|
Abdominal malfunction |
|
|
|
|
Gastroschisis |
|
|
|
|
Omphalocele |
|
|
| Airway obstruction | Choanal atresia |
|
Massive meconium aspiration |
| Upper airway | Pierre Robin syndrome |
|
Vocal cord paralysis secondary to myelodysplasia |
|
|
Laryngeal web/cleft |
|
|
|
|
Congenital tracheal/laryngeal stenosis |
|
|
|
|
Recurrent laryngeal palsy |
|
|
|
|
Hemangioma |
|
|
|
|
Lymphangioma |
|
|
| Lower airway | Tracheoesophageal fistula |
|
Meconium/blood aspiration |
|
|
Lobar emphysema |
|
|
| Alveolar disorders |
|
Respiratory distress syndrome | Bronchopulmonary dysplasia |
| Cardiovascular disorders | Congenital cardiac malformations |
|
Persistent pulmonary hypertension |
|
|
Congestive heart failure (critical coarctation or aortic stenosis) |
|
|
|
|
Total anomalous pulmonary venous return |
|
|
In summary, all causes of respiratory failure share similar pathophysiology, namely, atelectasis with decreased FRC and intrapulmonary right-to-left shunting or alveolar overdistention with increased dead-space volume and decreased CO2 elimination (or both). The concept of increased work of breathing is important to all causes of respiratory dysfunction because any clinical disease that causes increased work of breathing (i.e., decreased compliance or increased dead-space volume) can result in fatigue and a breathing pattern that further complicates the initial process.
The standard therapeutic approach to respiratory failure includes (1) maximizing the child's position, (2) increasing ambient oxygen, (3) relieving obstruction by either airway instrumentation or pharmacotherapy, (4) treating infection, (5) correcting fluid overload, (6) maximizing all nonpulmonary systems, and (7) instituting either negative-pressure or positive-pressure mechanical ventilation. More specialized and investigational forms of therapy should also be considered. Exogenous surfactant, high-frequency ventilation, lung-protective mechanical ventilation strategies, inhalation of NO, prone positioning, liquid ventilation, and extracorporeal membrane oxygenation may be effective therapies in particular clinical scenarios.
The child's position must be guarded. Unlike adults or older children, an infant may require help maintaining optimal positioning. Specific maneuvers may include keeping the child in a semi-upright position to avoid aspiration or gastroesophageal reflux, as well as minimizing the effects of abdominal distention. At times, the upper airway must be supported by keeping the head in a midline position and minimizing excessive neck flexion.
An increase in inspired oxygen can be accomplished in a number of ways. Tight-fitting anesthesia masks and nasal prongs may agitate the child and negate any beneficial effects. Blow-by oxygen, space masks, croup tents, and head boxes are alternative, less invasive maneuvers.
Relief of an upper airway obstruction can be lifesaving. Instrumentation of the airway includes placement of
| Impaired control of ventilation |
| Head trauma |
| Intracranial hemorrhage |
| Increased intracranial pressure secondary to tumor, edema, hydrocephalus, Reye's syndrome, etc. |
| Central nervous system infections |
| Ondine's curse |
| Drug intoxication |
| Status epilepticus |
| Neuromuscular disorders |
| High cervical cord injury |
| Poliomyelitis |
| Guillain-Barré syndrome |
| Neurodegenerative diseases (e.g., Werdnig-Hoffmann syndrome) |
| Muscular dystrophies and myopathies |
| Myasthenia gravis |
| Botulism |
| Tetanus |
| Phrenic nerve injury |
| Structural impairment |
| Severe kyphoscoliosis |
| Flail chest |
| Large intrathoracic tumor |
| Pneumothorax or pneumomediastinum |
| Large pleural effusion, hemothorax, empyema |
| Severe abdominal distention |
| Severe obesity (pickwickian syndrome) |
| Airway obstruction |
| Upper airway |
| Congenital anomalies |
| Tumor—intrinsic or extrinsic |
| Epiglottitis |
| Croup (laryngotracheobronchitis) |
| Foreign body |
| Postintubation edema, granulation tissue, or scarring |
| Vocal cord paralysis |
| Burns |
| Vascular ring |
| Lower airway |
| Asthma |
| Bronchiolitis |
| Foreign body |
| Lobar emphysema |
| Cystic fibrosis |
| Alveolar disorders |
| Pneumonia |
| Infectious—bacterial, viral, fungal, Pneumocystis |
| Chemical—aspiration, hydrocarbon, smoke inhalation |
| Pulmonary edema—cardiogenic, near-drowning, capillary leak syndrome |
| Massive atelectasis |
| Oxygen toxicity |
| Pulmonary contusion |
| Pulmonary hemorrhage |
Mechanical ventilation is the mainstay in the treatment of respiratory failure, and it provides respiratory support for nonpulmonary disease as well. The following are several nonpulmonary reasons for instituting respiratory support:
Mechanical respiratory assistance can be given by a variety of methods, including continuous positive airway pressure (CPAP), intermittent positive-pressure ventilation, and negative-pressure ventilation (see Chapter 75 ).[128] Positive pressure is applied almost exclusively by way of an intra-tracheal artificial airway, either an ETT or a tracheostomy tube. Oral intubations are easier to perform than nasal
The size of the ETT should be carefully selected. One formula
that estimates the required size of tube for children older than 2 years is as follows:
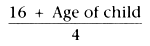
which gives the size by internal diameter. If the ETT size is correct, a slight
air leak should be present when a positive pressure of 20 to 30 cm H2
O
is applied to the airway.[129]
Serious lifelong
laryngeal and subglottic damage can result from insertion of an inappropriately large
ETT, particularly in children with inflammatory lesions of the upper airway such
as laryngotracheobronchitis. Because of the more flexible tracheal cartilage and
the relative subglottic narrowing in children, uncuffed ETTs generally provide an
adequate seal in those younger than 8 years. Small, cuffed ETTs are being used more
frequently in the ICU with the increased use of volume-controlled ventilators in
children. Cuffed tubes can eliminate the air leak around an ETT, but overinflation
of the cuff may result in airway injury.
After endotracheal intubation, the position of the tube within the trachea should be assessed. Physical examination should reveal symmetric chest movement and symmetric breath sounds on auscultation. An electronic or colorimetric CO2 detection system should be available to confirm the endotracheal position of the tube. On chest radiography, the tip of the ETT should be midway between the vocal cords and the carina. In small infants, such positioning leaves a very small margin of error before the tube has either progressed down one main stem bronchus or migrated above the vocal cords; extreme care should be taken to verify the position of the tube. It is important to realize that movement of the child's head and neck can affect placement of the tube. Flexion of the neck advances the tube farther into the trachea, and extension pulls the tube higher into the airway, thus making it important to avoid extreme flexion or extension of the neck during patient care.
In recent years, ETTs have been left in place in infants and children for increasing periods. It is not uncommon to leave a child's trachea intubated for more than 2 weeks and up to 12 weeks without performing a tracheostomy. This approach has been possible because of proper humidification and improved techniques of nursing care in both endotracheal suctioning and patient surveillance.
Despite these improved techniques, any child with an ETT in place must be constantly monitored for the complications of ETT obstruction by secretions and accidental extubation or accidental intubation of a main stem bronchus. Tracheostomy is indicated in children who require a long-term artificial airway for mechanical ventilation or endotracheal suctioning or to bypass obstruction of the upper airway. A potentially life-threatening complication of tracheostomy is accidental dislodgement of the tracheostomy tube soon after surgery before a well-healed track has formed. Reinsertion of the tube can be very difficult during these first 72 hours after surgery, and forceful blind reinsertion of a tracheostomy tube through a fresh tracheostomy site can result in blunt dissection between the tissue planes. The complications of airway obstruction, pneumomediastinum, and pneumothorax can result.
With CPAP, the child breathes spontaneously with a constant specified positive pressure applied to the airway. With PEEP, the child is ventilated with a constant specified end-expiratory pressure. Positive pressure during spontaneous inspiration is maintained by either placing a pressurized reservoir bag into the system or increasing fresh gas flow to exceed the inspiratory flow rate (gas flow rates of at least two to three times the patient's minute ventilation are required just to avoid rebreathing from the circuit). Exhalation occurs against a water column adjusted to deliver the desired pressure resistance.
CPAP is applied by way of an ETT, nasal cannulas, or facemask. Because newborns are obligate nose breathers, relatively low levels of CPAP can be produced with nasal cannulas. This method has had variable success, depending on the size and activity of the infant; crying and mouth breathing reduce the efficiency of the system. Gaseous distention of the abdomen is a common complication, and an orogastric tube is often required as a continuous vent.[130] CPAP through a facemask can be applied effectively to adults as well as infants and children for short periods. Mask CPAP has also been associated with the complications of gastric distention and pressure necrosis of the face and eyes.[128]
Low to moderate levels of PEEP can be maintained in children with an uncuffed ETT. If leakage around the ETT precludes a stable level of PEEP, two options are available: the ETT can be upsized to a tube with a larger internal diameter, or a cuffed ETT can be used.
The goals and risks of CPAP and PEEP are the same in children and adults. The optimal level of CPAP or PEEP can be difficult to ascertain for each clinical situation. Too little may afford no beneficial effect, whereas too much may cause overdistention of the lung and dead-space ventilation. Low levels of CPAP or PEEP (2 to 5 cm H2 O) are often considered homeopathic and have been advocated by some authors for any child with an artificial airway in order to maintain FRC at preintubation levels.[131]
In children with lung disease, the goal of optimal CPAP or PEEP is to maximize improvement while minimizing side effects. A conservative approach to choosing pressure is to limit CPAP to levels sufficient to improve oxygenation by decreasing FIO2 to 0.6 or less while the patient experiences no cardiovascular changes. A second approach is to increase pressure levels until maximal improvement in compliance is achieved. A bedside measurement of compliance can be calculated in ventilated patients to assess the effect of PEEP. Airway pressure is measured at the end of a 3- to 5-second inspiratory hold. Compliance is then estimated by subtracting the end-expiratory pressure from this end-inspiratory hold pressure and then dividing by the measured expired tidal volume. End-inspiratory hold pressure is used rather than peak airway pressure because it is a more accurate assessment
Suter and colleagues[133] proposed that the optimal or best PEEP (or CPAP) is the level of pressure associated with maximal oxygen transport, which is defined as the product of cardiac output and arterial oxygen content. This concept accounts for both the beneficial effect of improved oxygenation and the complication of decreased cardiac output. This is important in view of the finding that once a patient is receiving higher than optimal PEEP, airway pressure is more directly transmitted to the cardiovascular system and may decrease cardiac output and, hence, oxygen transport. To apply Suter and associates' concept of best PEEP, repeated cardiac output determinations are required, thus necessitating a Swan-Ganz thermodilution catheter. The indications and risks of pulmonary artery catheterization must be weighed against the benefits of accurately determining the best PEEP and the risks of high levels of pressure. Most clinicians accept that an approximation of optimal CPAP or PEEP can be safely determined by clinical trial and compliance estimates, as long as the level of CPAP or PEEP is less than 20 cm H2 O. If more than 20 cm H2 O is being considered, thermodilution catheters are often inserted.
Another alternative for estimating the CPAP or PEEP level is the use of an esophageal pressure monitor. Ideally, esophageal pressure should change only slightly until optimal intrathoracic pressure is reached. Once the maximal improvement in lung compliance is attained, increasing CPAP will result in greater transmission of pressure to the esophagus.
Positive-pressure mechanical ventilators can be classified according to their method of control as (1) volume preset, (2) pressure preset, and (3) time-flow preset.[134] As a general rule, time-flow- or pressure-preset ventilators are more convenient to use in infants and young children (<10 kg), whereas volume-preset ventilators are most commonly used in older children (>10 kg) and adults. Time-flow- and pressure-preset ventilators have several advantages in ventilatory assistance in infants and young children. Most commonly, infants and young children are intubated with an uncuffed ETT, so a variable amount of leak is produced around the ETT. This leakage, along with the relatively large compression volume factor in relation to tidal volume in infants, makes the volume-preset determination on volume ventilators unreliable.[135] [136] The main problem with using any pressure-preset or time-flow-preset ventilator is that the volume delivered depends on the child's total thoracic and lung compliance. Consequently, overventilation and an increased risk of alveolar rupture may occur as compliance increases. Alternatively, it may cause hypoventilation should compliance decrease.
Intermittent mandatory ventilation (IMV) is available on pressure-, time-flow-, and volume-preset ventilators.[137] [138] In the IMV system, the child may breathe spontaneously from a fresh gas source at low resistance while receiving active inflation from the mechanical ventilator at preset intervals with the same level of end-expiratory pressure. This system allows the child additional breaths at the same FIO2 and PEEP. IMV is produced either by a continuous-flow circuit or by a series of demand valves. Continuous-flow IMV circuits are simpler and do not require additional patient effort during spontaneous breathing. Thus, continuous-flow circuits may have an advantage over demand-valve systems in children with relatively high respiratory rates, in whom valve sensitivity and response time are potentially important during weaning from mechanical ventilation. Pressure support ventilation is an innovation of the assist/control mode of ventilation. In pressure support ventilation, each spontaneous breath is augmented by the delivery of gas at a preset pressure. The patient determines the respiratory rate and the inspiratory time. The pressure support increases the delivered tidal volume and may decrease the work of breathing and improve patient comfort. This mode is most commonly used while weaning a patient from mechanical ventilation. Because it depends on the patient's own initiation of a breath, pressure support cannot be used in a patient who has an abnormal respiratory drive.[139] [140]
The pressure pattern of ventilation is dependent on the inspiratory and expiratory flow rates, tidal volume, the duration of inspiration and expiration (I/E ratio), and the respiratory rate. The optimal ventilatory pattern varies from patient to patient and from one disease state to another.
Insight into the pathophysiology of the pulmonary interstitial or alveolar disease represented by acute respiratory distress syndrome (ARDS) has led to better understanding of the implications of ventilator management. Table 76-10 outlines the salient features of the pathophysiology of ARDS. This knowledge has led to a change in therapy to the use of ventilator strategies that involve relatively long inspiratory times, high end-expiratory pressure, and low tidal volume. This technique results in lower minute ventilation and elevated PaCO2 . Termed lung-protective ventilator strategy, this technique decreases the shear forces acting on the terminal airways and recruits lung units that have dropped below closing volume.[141] Acid-base status must be maintained with a compensatory metabolic alkalosis that occurs gradually through renal retention of bicarbonate. This process can be assisted by administering sodium bicarbonate or tromethamine and allowing time for equilibration of blood tissue pH.
By contrast, the optimal pattern of ventilation for patients with
obstructive airway disease is different. In these patients, using a faster inspiratory
flow rate with
| Disruption of endothelial barriers with leakage of protein-containing fluids and inflammatory cells into the alveoli |
| Inactivation of surfactant resulting in increased surface tension of the alveolar lining layer |
| Atelectasis and lung volume loss |
| Shunting of blood through nonventilated areas of lung resulting in hypoxemia and tissue hypoxia |
| Decreased pulmonary compliance |
Despite the lack of a specific formula for initiating mechanical ventilation, some general principles can be followed. In our experience, the human hand is still the best mechanical ventilator. After a patient has been intubated and stabilized, hand ventilation with a Mapleson-type anesthesia circuit with an airway pressure manometer in line is useful in determining the approximate pressure required to inflate the chest. In patients with alveolar or interstitial disorders, a prolonged I/E ratio of 1.5:1 or 1:1 is frequently useful, with a relatively slow frequency of less than 24 cycles/min in an infant and less than 16 cycles/min in a child.
When initiating mechanical ventilation with a volume-preset ventilator, an arbitrary tidal volume of 10 to 15 mL/kg (using the higher number in a smaller child) while observing peak airway pressures is commonly chosen. Regardless of the type of ventilator, the most important variables in initiating mechanical ventilation are the adequacy of chest expansion and gas exchange by clinical observation and auscultation and the adequacy of alveolar ventilation by measuring PaCO2 . Peak airway pressure should be measured frequently as close to the ETT as possible.
PEEP should begin at 3 to 4 cm H2 O until arterial oxygenation is maximized. It is unusual to require end-expiratory pressure above 20 cm H2 O in pediatric patients.
With the initiation of positive-pressure ventilation, it is not uncommon to see systemic arterial hypotension, which usually responds to a volume infusion of 10 to 20 mL/kg of crystalloid or colloid. However, it is strongly recommended that CVP be measured in any patient requiring mechanical ventilation with PEEP of 10 cm H2 O or greater. These data are helpful in determining the effects of mechanical ventilation and positive airway pressure on the cardiovascular system.
At least initially, some degree of sedation is required for conscious children to cooperate with mechanical ventilation. The amount of sedation depends on the child's age, size, underlying disease, and degree of respiratory support required. Occasionally, an infant in respiratory failure may be initially severely depressed and not require any sedation. Sedation facilitates the patient breathing "in phase" with the ventilator so that minimum peak airway pressure can be maintained without coughing and straining. It is not unusual to find that once the child accommodates to the mechanical ventilator, the amount of sedation required can be reduced. Various combinations of sedatives have been advocated for children, with probably equal success. The drug that I usually administer is fentanyl (1 to 2 μg/kg/hr), given intravenously as a constant infusion after an initial bolus. If additional sedation is required, one may use lorazepam (0.1 to 0.3 mg/kg), administered intravenously every 4 to 6 hours, or midazolam (0.05 to 0.2 mg/kg/hr) given as an infusion. These drugs have minimal cardiovascular effects, provided that intravascular volume is adequate.[142]
There is no perfect pharmacologic recipe for chronic sedation in the ICU. Unfortunately, because children experience tachyphylaxis to many of these drugs, the dosage is continuously increased or other agents are added, or both. The result can be a polypharmacy of extremely high drug doses, and weaning from this sedation adds to the patient's problems. Some children have symptoms of withdrawal and require a very slow drug taper, which is often accomplished by switching to oral agents such as methadone and lorazepam.
Neuromuscular blocking drugs are used to increase chest wall compliance, reduce oxygen consumption,[143] and more commonly, facilitate mechanical ventilation by preventing patient-ventilator dyssynchrony. These drugs should always be used in combination with medications that provide amnesia and anxiolysis. Adequate analgesia should be provided to patients to ensure that pain is well controlled.
Pancuronium and vecuronium are used most commonly in the PICU (also see Chapter 13 ). The usual dose of pancuronium is 0.1 mg/kg administered intravenously every 1 to 1½ hours or 40 to 100 μg/kg/hr as an infusion. Although tachycardia with pancuronium is an undesired side effect[144] in adults, it is rarely of clinical significance in infants and children. Vecuronium and cisatracurium are competitive blocking agents with a shorter duration of action than pancuronium. Vecuronium (0.08 to 0.2 mg/kg, followed by an infusion of 60 to 150 μg/kg/hr) causes less tachycardia than pancuronium does; cisatracurium (0.1 to 0.2 mg/kg, followed by an infusion of 60 to 120 μg/kg/hr) is not dependent on renal or hepatic function for elimination. Neuromuscular blocking agents need to be titrated with the use of a peripheral nerve stimulator or by observing movement, with regular drug holidays.
The criteria for weaning are ill-defined clinical factors. In general, weaning from respiratory support should begin only when the child has a stable cardiovascular system and is awake and alert. It is unwise to begin reducing respiratory support in a child who is still at risk for acute cardiac decompensation. It is also best to wait until other major metabolic abnormalities have been corrected. Severe anemia, hypoglycemia, hypernatremia, hypochloremia, or malnutrition may severely impair a child's ability to be weaned from mechanical ventilation. The chest wall and diaphragm must be intact, and the child should be able to generate at least 20 cm H2 O pressure at the airway (inspiratory force) and move at least 10 mL/kg of air with maximal effort (vital capacity). The parameters that can then be weaned include rate, FIO2 , peak inspiratory pressure, and CPAP/PEEP.
On pressure-limited or time-flow-limited ventilators, the peak inspiratory pressure must be reduced because compliance improves during the weaning process. The best indicators of a change in compliance are the degree
Ventilator rates should not be weaned until the ABGs are stable with a required inspired oxygen concentration of less than 0.5, a required PEEP of less than 10 cm H2 O, and a peak airway pressure of less than 30 to 35 cm H2 O.
One must also be certain that all residual effects of neuromuscular blocking drugs have disappeared and that a reduced level of sedation is ordered. Neuromuscular blockade can be reversed with the intravenous administration of neostigmine (0.05 mg/kg) and glycopyrrolate (0.01 mg/kg); a peripheral nerve stimulator can confirm the presence of acceptable neuromuscular function. Weaning is then begun by gradually decreasing the ventilator rate over a period of hours to days.
Weaning should continue as long as the ABGs remain within an acceptable range and the child's clinical condition tolerates weaning. As the amount of effective spontaneous ventilation increases, the increased work of breathing may cause the child's overall condition to change before blood gases begin to deteriorate. Tachycardia, hypertension or hypotension, tachypnea and increased work of breathing, and anxiety are danger signs. Weaning should be discontinued and additional ventilatory support reinstated. Frequent assessment of ABGs and observation of the child's clinical condition are essential throughout the weaning process. If a child has any residual lung disease with decreased lung compliance, there may be a tendency for the development of decreased FRC with atelectasis and decreased lung volumes. These potential problems can be minimized by weaning on moderate levels of CPAP or PEEP (5 to 10 cm H2 O).
Extubation should be performed by an individual skilled in laryngoscopy should gas exchange deteriorate and reintubation be required. Once tracheal extubation has occurred, it is not unusual for the patient to require a 20% increase in FIO2 by facemask or Oxy-Hood oxygenator. It is essential that the patient be encouraged to cough and clear secretions while taking slow, deep breaths as often as possible. Incentive spirometry, early mobilization, and chest physical therapy play a significant role in recovering from respiratory failure.[145] [146]
It should be emphasized that weaning from mechanical ventilation requires close clinical observation, frequent assessment of blood gases, and good clinical judgment. The rate of weaning depends on the child's overall clinical condition, as well as the underlying pathologic process. A child who is ventilated postoperatively overnight may be weaned very rapidly (over an hour or two), whereas a child recovering from severe interstitial pneumonia may require days to weeks to be weaned successfully. In chronically ventilated patients, meticulous attention must be paid to problems in other systems. Intravascular fluid balance must be excellent, with the patient kept relatively "dry." Serum bicarbonate and chloride should be kept in the normal range to prevent compensatory hypercapnia. The hematocrit must be maintained in the high-normal range to minimize cardiovascular stress. Caloric intake should be maximized with enteral or intravenous alimentation. Infection must be under control, and it is best if the child can be alert and cooperative. If the weaning process is stressful, rest periods of full-time mechanical ventilation, particularly at night, may be helpful. We all find a difficult task more easily handled after a good night's sleep.
Before tracheal extubation is considered, careful assessment of the quality and volume of endotracheal secretions is essential. Large, thick secretions may cause extubation to fail if the child is unable to cough and clear the secretions adequately. Extubation should be planned carefully and should preferably be performed in the morning while a full staff is available for careful observation. If the weaning and extubation process has been carefully planned and executed, reintubation should be a rare occurrence.
Body-type negative-pressure ventilators, first described by Drinker and Shaw in 1929[147] and used extensively during the polio epidemic of the 1950s,[148] have achieved renewed popularity. Reports of these devices in children and adults with chronic respiratory insufficiency have rekindled interest in the mechanics and physiology of negative-pressure ventilation. Patients with severe neuromuscular disabilities, such as Duchenne's muscular dystrophy, and those with chronic obstructive pulmonary diseases, such as cystic fibrosis, have been reported to have sustained improvement in their gas exchange and general clinical condition when treated by chronic or nighttime home ventilation. Measurements of vital capacity, inspiratory force, and the force of diaphragmatic muscle contraction suggest in a preliminary way that this therapy will improve gas exchange, reduce the complications of chronic hypoxemia and hypercapnia, and prolong life.[149]
Several negative-pressure ventilators are available commercially. The iron lung is most commonly used in the hospital setting. Its large size and weight, as well as its cost, are limitations to home use. The portable body cuirass and raincoat have achieved popularity for home use. An intermittent vacuum pump provides suction by producing a negative pressure within the body shell or cuirass. Though portable and reasonably affordable, these devices are less efficient and noisy and require closer attention because of the difficulty of achieving a tight seal and the likelihood of air leaks developing with body movement. Continued use and future critical study will be important in determining the role of negative-pressure ventilation in the treatment of respiratory failure.
The main advantage of negative-pressure ventilation is that the airway does not have to be instrumented with an ETT or a tracheostomy tube. Unfortunately, it has numerous serious clinical limitations. For example, patient size and patient deformity can make the patient-machine interface both difficult and uncomfortable, and the ability to provide basic and specialized patient care can be compromised. Any degree of upper airway obstruction (anatomic/structural or massive secretions) decreases the effectiveness of gas flow in negative-pressure ventilation. Finally, negative-pressure ventilation is not effective in patients with very poor lung compliance; maximal respiratory pressure is 30 cm H2 O, and it is not possible to sustain a reliably constant distending airway pressure
High-frequency ventilation describes a pattern of ventilation characterized by smaller than anatomic dead-space volumes delivered at high respiratory rates (>150 breaths/min). High-frequency ventilation can be generated by a number of different types of ventilators: the high-frequency jet ventilator, the high-frequency oscillating ventilator (HFOV), and the flow interrupter. Each of these machines differs in technical design and clinical application and most likely differs in the mechanics of gas exchange.[150]
Use of the HFOV has become accepted practice for neonatal patients with respiratory failure. There is impressive evidence that use of the HFOV has successfully supported infants who would previously have required extracorporeal membrane oxygenation (ECMO) or who would have died.[151] [152] HFOV has been successful in treating children with acute homogeneous interstitial and alveolar disease.[153] The use of HFOV in large children and adults has been less successful to date because of physical limitations of the equipment; however, ongoing research suggests that the utility of this mode will be expanded. Jet ventilation is used in some centers for multiple causes of respiratory failure, although the main indication remains a patient with barotrauma.
The administration of exogenous surfactant is now routine therapy for a surfactant-deficient premature infant. It has clearly improved the survival of these children and decreased their need for aggressive mechanical support.[154] Exogenous surfactant has not enjoyed the same success in older children and adults. The primary problem is the nature of the lung disease. Whereas a premature infant has an inadequate amount of surfactant, an older patient has surfactant dysfunction or inhibition rather than an absolute decrease in amount. Therefore, exogenous surfactant is vulnerable to the same inhibition.
Perfluorochemical (PFC) liquids are clear, colorless fluids approximately twice as dense as water with one fourth its surface tension, 16 times the oxygen solubility, and 3 times greater CO2 solubility. PFC liquid was first used as a respiratory medium in the 1960s[155] and was popularized in the 1989 movie The Abyss. The first human trials of tidal PFC liquid ventilation involved three preterm infants "at the point of death" because of severe respiratory failure. They were successfully ventilated for two 3- to 5-minute cycles by using PFC liquid tidal volumes exchanged by gravity.[156]
More recent approaches have used PFC liquid-filled lungs and gas ventilation with a conventional mechanical ventilator. This therapy is referred to as partial liquid ventilation (PLV) or perfluorocarbon-associated gas exchange. [157] The PLV technique has been shown to produce significant improvement in lung function and viability in numerous animal studies of diseased or injured lungs. The decreased surface tension of the perfluorocarbon-gas interface enables recruitment of atelectatic alveoli with lower ventilator pressures. Prospective, randomized, controlled pilot studies in adult and pediatric patients with acute lung injury or ARDS have not demonstrated a benefit of PLV over conventional ventilation, and it is unlikely that PLV will play a general role in the treatment of adult or pediatric ARDS.[158] Perfluorocarbons instilled into the lungs may play a role in treating ECMO-dependent neonates with congenital diaphragmatic hernia.[159]
ECMO is standard of care for term (34 weeks' gestation) neonates with acute respiratory failure (ARF). More than 11,000 infants with an 80% predicted mortality rate with conventional management have received ECMO support, 81% of whom survived.[160] Although neonatal ECMO is successful, the technology and the patient populations are constantly changing. The majority of ECMO is venoarterial (VA), in which blood is drained from the venous system and returned to the ascending aorta, thereby supporting not only respiratory but cardiac function as well. Venovenous (VV) ECMO is less efficient than VA ECMO, but it preserves pulmonary flow and avoids cannulation of the arterial system. VV ECMO is rapidly gaining popularity, and many clinicians believe that VV ECMO will be more commonly used than VA ECMO.[161] In addition to these technical changes, the target neonatal ECMO populations are also changing. Exogenous surfactant and new modes of ventilation such as HFOV are "saving" children from ECMO. In fact, most ECMO centers report that their ECMO candidates are a sicker group of infants with a greater preponderance of sepsis and multiorgan system failure. ECMO support for premature infants is also being evaluated in some centers by phase I studies. [162]
ECMO support for an older child/adult with ARF continues to pique interest. Approximately 500 pediatric ARF ECMO patients have been reported to the Extracorporeal Life Support Organization. All were patients who had a presumed 80% to 100% predicted mortality. With ECMO support, 52% have survived.[160] These data are difficult to evaluate in this population because of the extreme heterogeneity in age, diagnoses, conventional management, and criteria for ECMO. In addition, whereas the neonatal causes of ARF generally have reversible pathophysiologic features, reversibility of ARF in the older patient population is uncertain.[163] [164]
|
|
|
|
|
|
|
|
|
|
|
|
|