|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A major toxic effect of HBO therapy is due to the O2 itself. In fact, O2 toxicity is the factor that limits the maximum allowable PO2 and duration of hyperbaric treatment. Current opinion is that O2 toxicity is caused by excessive production of oxygen free radicals (e.g., superoxide, hydroxyl radicals, and singlet oxygen). Mechanisms within the body to scavenge these free radicals may be overcome by increased rates of free radical production at high O2 partial pressures.[149] With the use of supplemental O2 at 1 ATA, the manifestations of O2 toxicity are almost exclusively confined to the lung.
O2 toxicity during HBO therapy affects mainly three organ systems: the lung, the CNS, and the eye. Pulmonary toxicity in a conscious patient is heralded by symptoms of tracheobronchial irritation, namely, cough and burning chest pain. Prolonged exposure may result in a decrease in vital capacity (VC), ARDS, and ultimately,
 Figure 70-10
Decrease in vital capacity (VC) as a function of time
breathing 100% O2
at 2 ATA in humans. The figure illustrates the value
of intermittent O2
(20 minutes of O2
, 5 minutes of air) versus
continuous administration in the prevention of pulmonary O2
toxicity.
Numbers in parentheses represent the number of subjects tested. (Redrawn
from Clark JM: Oxygen toxicity. In Bennett PB,
Elliott DH [eds]: The Physiology and Medicine of Diving. Philadelphia, WB Saunders,
1993, p 121.)
Figure 70-10
Decrease in vital capacity (VC) as a function of time
breathing 100% O2
at 2 ATA in humans. The figure illustrates the value
of intermittent O2
(20 minutes of O2
, 5 minutes of air) versus
continuous administration in the prevention of pulmonary O2
toxicity.
Numbers in parentheses represent the number of subjects tested. (Redrawn
from Clark JM: Oxygen toxicity. In Bennett PB,
Elliott DH [eds]: The Physiology and Medicine of Diving. Philadelphia, WB Saunders,
1993, p 121.)
The degree to which O2
is toxic is related to the PO2
of the inspired gas. At 1 ATA, 100% O2
is as toxic as 16.7% O2
at 6 ATA or 2% O2
at 50 ATA. An attempt to quantify O2
exposure
was made by Clark and Lambertsen.[150]
In this
system (unit pulmonary toxic dose [UPTD]), the number of UPTD units (U) is calculated
by the formula
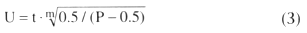
where t is the exposure time in minutes, P is the inspired PO2
in ATA, and m is a slope constant that has an empirical value of 1.2. After 1425
UPTD units of O2
exposure, VC decreases an average of 10%; after 2190
UPTD units, there is an associated decrease of 20%. Complete reversal of VC decrements
as large as 40% of control has been observed after extended O2
exposure
at 2 ATA.[150]
Reanalysis of a larger data set than the one used for the UPTD
model, but which included these data, resulted in a different prediction equation:
%ΔVC = −0.009 · (P − 0.38) ·
t (4)
where P and t are the same as for Equation 3.[151]
Using previously published data, Arieli[151A]
developed the following equation:
%ΔVC = 0.0082 · t2
(PO2
/101.3)4.57
(5)
where t is time in hours and PO2
is pressure
in kilopascals (kPa).
Although these algorithms may be useful as an approximate guide to safe O2 exposure in populations, interindividual variability is such that they cannot be relied on to accurately predict the development of pulmonary O2 toxicity. In one study, 12 healthy men who breathed gas containing a PO2 equal to 0.3 ATA for 12 hours and then a PO2 equal to 1.05 ATA for 48 hours experienced decrements in VC ranging from 0% to nearly 30%.[152] Furthermore, O2 toxicity can be modified by humidity,[153] circulating catecholamine and corticosteroid levels, leukocyte accumulation in the lungs (e.g., with pneumonia), and circulating endotoxin. A more useful guide in practice is the patient's symptoms. Early symptoms of pulmonary oxygen toxicity include cough and central burning chest pain that increases with deep inspiration. These symptoms do not occur during routine repetitive HBO treatments but may become evident during extensive O2 periods at 2.8 ATA, for example, when treating neurologic decompression illness. Asymptomatic persons usually have minimal or no change in VC. The minor changes in forced expiratory volume in 1 second (FEV1 ) reported during repetitive HBO treatments[154] are of uncertain clinical importance.
Some of the effects of pulmonary O2 toxicity can be cumulative. Patients given supplemental O2 to breathe between hyperbaric treatments often complain of burning chest pain during HBO. Therefore, in patients receiving multiple HBO treatments, between hyperbaric exposures it is best to use the minimum inspired O2 compatible with clinical safety (e.g., in the intensive care unit).
Some antineoplastic drugs such as bleomycin[155] and mitomycin C[156] [157] appear to predispose to fatal pulmonary O2 toxicity (ARDS and respiratory failure) from what should otherwise have been well-tolerated doses of supplemental O2 . The risk of pulmonary O2 toxicity from HBO therapy in patients with previous exposure to such drugs is unknown, although we have treated several such individuals with a remote history of bleomycin treatment with repetitive doses of HBO. In some of these individuals, mild pulmonary O2 toxicity symptoms did develop, such as retrosternal chest tightness, but none experienced
CNS O2 toxicity is manifested in its most severe form as nonfocal tonic/clonic seizures. It may occur without warning but is sometimes preceded by premonitory signs or symptoms (e.g., nausea, facial numbness, facial twitching, bradycardia, unpleasant olfactory or gustatory sensations, acoustic symptoms). The probability of seizures increases with increasing PO2 and time of exposure. In a study of 36 divers breathing 100% O2 at 3.7 ATA, all experienced one or more of these symptoms within 100 minutes or less.[158] [159] In clinical practice, convulsions in patients undergoing HBO therapy are rare at ambient PO2 up to 2.5 ATA (typically 0.008% to 0.035% of treatments[160] ), and these patients often have another predisposing factor such as hypoglycemia. The probability of a convulsion is greater at higher PO2 and when administered for acute indications such as CO poisoning.[161] Metabolic factors may reduce the seizure threshold, such as the administration of high-dose penicillin (e.g., for clostridial infection), sepsis, and hypoglycemia.
Treatment of hyperoxic seizures is easily accomplished by immediately decreasing the inspired O2 concentration, which in a multiplace chamber can be achieved by removing the head tent or facemask, and allowing the patient to breathe air. An intubated patient can be manually ventilated with a reduced FIO2 until the seizure has stopped. In a monoplace chamber, 100% O2 in the chamber atmosphere may be replaced by air. The chamber should not be decompressed while the patient is actively having a seizure because airway closure and failure to exhale during this period may result in pulmonary barotrauma such as pneumothorax or AGE.
Some hyperbaric physicians routinely administer an anticonvulsant such as phenobarbital, phenytoin, or a benzodiazepine after a hyperoxic seizure, although others do not believe that an anticonvulsant is necessary. Other than pulmonary barotrauma (which can occur if chamber decompression is performed during a seizure, as noted earlier), hyperoxic seizures have no sequelae and rarely recur despite continuation of HBO, particularly if anticonvulsant prophylaxis is used. Thus, hyperoxic CNS symptoms should not be a reason to discontinue a series of HBO treatments.
Hyperoxic effects on the eye may be acute (narrowing of the visual fields) or chronic (change in the refractive index of the lens resulting in myopia). These changes occur during the course of intermittent HBO therapy over a period of several weeks and usually resolve in a similar time frame. Nonetheless, some patients may be left with residual myopia, particularly the elderly.[162] It has been suggested that hyperbaric therapy may promote nuclear cataract formation. [163] However, the population of patients most likely to require hyperbaric treatment (elderly people with peripheral vascular disease and diabetes) also have a high prevalence of cataracts. Moreover, cataractous lenses tend to progress without hyperbaric exposure, and the changes are difficult to quantify, as pointed out by Anderson and Shelton.[162] Therefore, more definitive data are required before the hypothesis that HBO may result in cataracts can be accepted. Additional toxic effects of O2 on the neonatal retina have been well described and may result in retrolental fibroplasia. Concern has been raised about the risk of this complication in the unborn child of a woman who may require acute HBO therapy during pregnancy. Although many pregnant women have been treated with a single exposure to HBO (e.g., for CO poisoning), we are not aware of any incidents of retrolental fibroplasia in the child after birth. We therefore recommend that pregnant women with an acute, symptomatic illness for which HBO is indicated (e.g., CO poisoning) be treated with HBO because the risk to the fetus of the underlying condition is usually much greater.
O2 toxicity of other organs has been suggested or demonstrated in experimental animals, but its importance has not become evident, at least with currently used HBO schedules.
|
|
|
|
|
|
|
|
|
|
|
|
|