|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
After the possibility of endotracheal tube ignition was raised, extrinsic protection of the tubes was suggested.[73] Patil and colleagues[74] suggested wrapping tubes with moistened muslin. However, if allowed to dry, muslin becomes flammable. Kumar and Frost[75] suggested coating the vulnerable portion of the exterior of the tube with dental acrylic. This approach, however, renders the tube rigid with a rough surface, which may traumatize the mucosal surfaces. The most popular approach to the problem has been wrapping the tube with metallized foil tape.[50] [59] [61] [63] [73] [76] [77]
Three types of tape have been used: aluminum foil with adhesive backing, copper foil with adhesive backing, and plastic tape thinly coated with metal on one side and adhesive on the other. These tape products are widely available from electronics, arts and crafts, and building supply shops. Lead foil (commonly used on windows for burglar alarms) is similar in appearance but is toxic and should never be used in the airway.
The aluminum and copper tapes have been evaluated for protection of different types of endotracheal tubes with various lasers. Although taping does not provide protection of the inflatable cuff portion of an endotracheal tube, these metal tapes do offer a measure of protection
Applying a protective metal foil wrap to an endotracheal tube requires some care. A clean tube should be wiped with alcohol to remove residue that could interfere with adhesion and then optionally wiped lightly with Mastisol or tincture of benzoin. The end of the tape should be cut at an angle of about 60 degrees and the cut edge aligned with the proximal end of the cuff junction ( Fig. 67-8 ). Tape should be wrapped in a spiral, with about 30% overlap, to the exit point of the cuff pilot tube. Wrinkles should be eliminated to avoid abrading the tracheal mucosa. The result should eliminate bare tubing or areas of exposed tape adhesive (which is quite flammable). Rewiping the wrapped tube with alcohol provides a degree of cleanliness before tracheal intubation.
Although this use is well supported in the medical literature, none of these metal foils has FDA approval because their manufacturers have not sought it. Physicians who devise a wrapped endotracheal tube using such tapes incur some product liability risk as a noncertified "manufacturer" should injury occur, especially because there are FDA-approved commercial products which claim laser resistance.
The Merocel Laser Guard (Medtronic Xomed Merocel Corp., Mystic, CT) is a commercial, FDA-approved endotracheal wrap consisting of an adhesive metal foil laminated to a synthetic sponge surface. Properly placed on an endotracheal tube and kept moist, the Merocel product provides protection against CO2 , argon, and KTP:Nd:YAG but not YAG lasers.[78] The manufacturer recommends this product only for use with CO2 lasers. The shield material adds almost 2 mm to the diameter of the endotracheal tube. Like the nonapproved tapes, Laser Guard can be applied only to the shaft of the tube and provides no protection for the cuff.
The FDA also has approved the use of an integral laser-resistant coating in the manufacture of endotracheal tubes. The commercially available Xomed Laser shield tube (Medtronic Xomed, Jacksonville, FL) is fabricated from
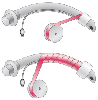 Figure 67-8
Cuff wrapping technique. If a wrapped endotracheal tube
is the chosen method for laser protection, the technique for wrapping is critical
in ensuring protection from ignition and foil-induced mucosal abrasions. It is often
helpful first to paint the tube sparingly with a medical adhesive such as benzoin
or Mastisol. The end of the tape should be cut with a scalpel to approximately 60
degrees. Wrapping is begun by aligning the cut end of the tape with the junction
of the tube and the proximal end of the cuff and is done in a spiral with a 30% to
50% overlap between layers. Wrapping should include the inflation tube for the cuff
and should be continued until just short of the pilot balloon, with care taken not
to wrinkle the tape at any point.
Figure 67-8
Cuff wrapping technique. If a wrapped endotracheal tube
is the chosen method for laser protection, the technique for wrapping is critical
in ensuring protection from ignition and foil-induced mucosal abrasions. It is often
helpful first to paint the tube sparingly with a medical adhesive such as benzoin
or Mastisol. The end of the tape should be cut with a scalpel to approximately 60
degrees. Wrapping is begun by aligning the cut end of the tape with the junction
of the tube and the proximal end of the cuff and is done in a spiral with a 30% to
50% overlap between layers. Wrapping should include the inflation tube for the cuff
and should be continued until just short of the pilot balloon, with care taken not
to wrinkle the tape at any point.
Although more resistant to far-infrared radiation than PVC or red rubber tubing,[79] [80] the Laser Shield nonetheless burns vigorously once ignited in vitro,[63] producing friable silica dust. It also may ignite under typical surgical conditions, thereby failing the ECRI flammability test.[78] In at least one case, an airway fire involving this tube occurred,[56] resulting in serious injury attributed to an intraluminal fire caused by laser penetration of the cuff; the laser power settings were within specified limits, but the respiratory gas mixture included an excessive FIO2 and nitrous oxide. A newer version, the Laser Shield II, is approved for use with CO2 or KTP lasers. The construction of this version includes a silicone-based tube, which is smoothly wrapped by a coated aluminum tape. The cuff is made of unshielded silicone elastomer, designed to be expanded with saline, and includes methylene blue dye to tint the saline. The tube wrap is specified to withstand (in vitro) 35,000 W/cm2 of CO2 laser energy or 11,000 W/cm2 of KTP energy for up to 3 minutes. Blood or
|
|
|
|
|
|
|
|
|
|
|
|
|